Recombinant human collagen containing hydroxyproline and hydroxylysine and production method of recombinant human collagen
A technology of human-like collagen and hydroxylysine, applied in the direction of animal/human protein, biochemical equipment and methods, peptide/protein components, etc. To achieve the effect of promoting cell proliferation and promoting adhesion, accelerating wound hemostasis and healing, and promoting the repair of epidermal cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Recombinant Human Collagen Nucleotide and Amino Acid Sequence Design, Construction of Expression Vector
[0041] Based on the codon preference of Kluyveromyces, the recombinant human-like collagen (hlCOL), α subunit of proline hydroxylase (P4H), and Protein disulfide isomerase (PDI), lysine hydroxylase L230 (the code name of lysine hydroxylase L230 is LH) protein gene hlCOL, P4HA, PDI, LH. The gene sequence was synthesized by Shanghai Jierui Bioengineering Co., Ltd. The nucleotide sequence of the synthetic recombinant human-like collagen (hlCOL) is shown in SEQ ID No.1, the α subunit of proline hydroxylase (P4H) The nucleotide sequence is shown in SEQ ID No.2, the nucleotide sequence of lysine hydroxylase is shown in SEQ ID No.3, and the nucleotide sequence of lysine hydroxylase is shown in SEQ ID No.4. Obtained from the nucleotide sequence encoding expression of recombinant human-like collagen (hlCOL), α subunit of proline hydroxylase (P4H), protease disulfid...
Embodiment 2
[0052] Example 2: Construction of hydroxylated collagen-like engineering bacteria
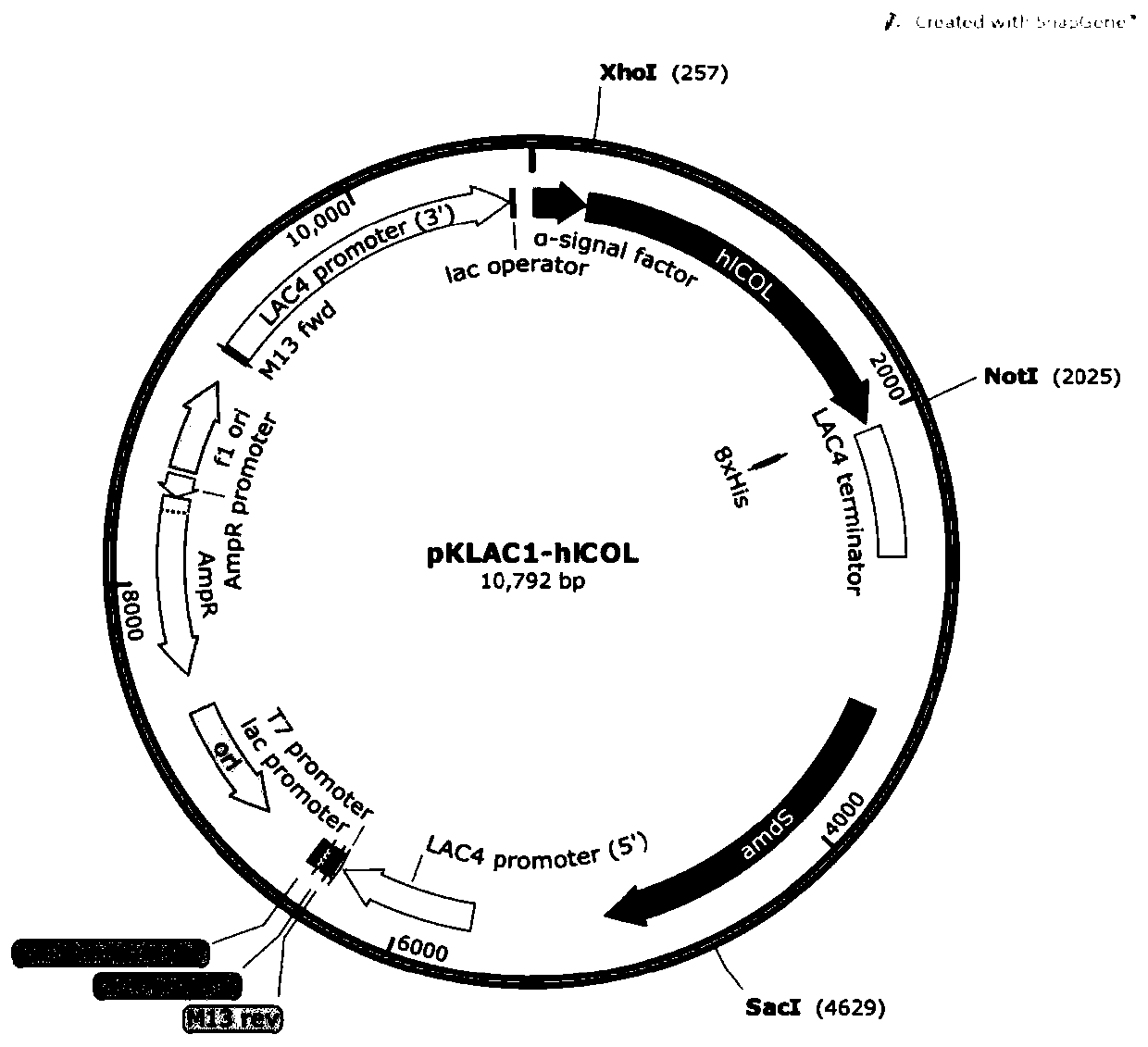
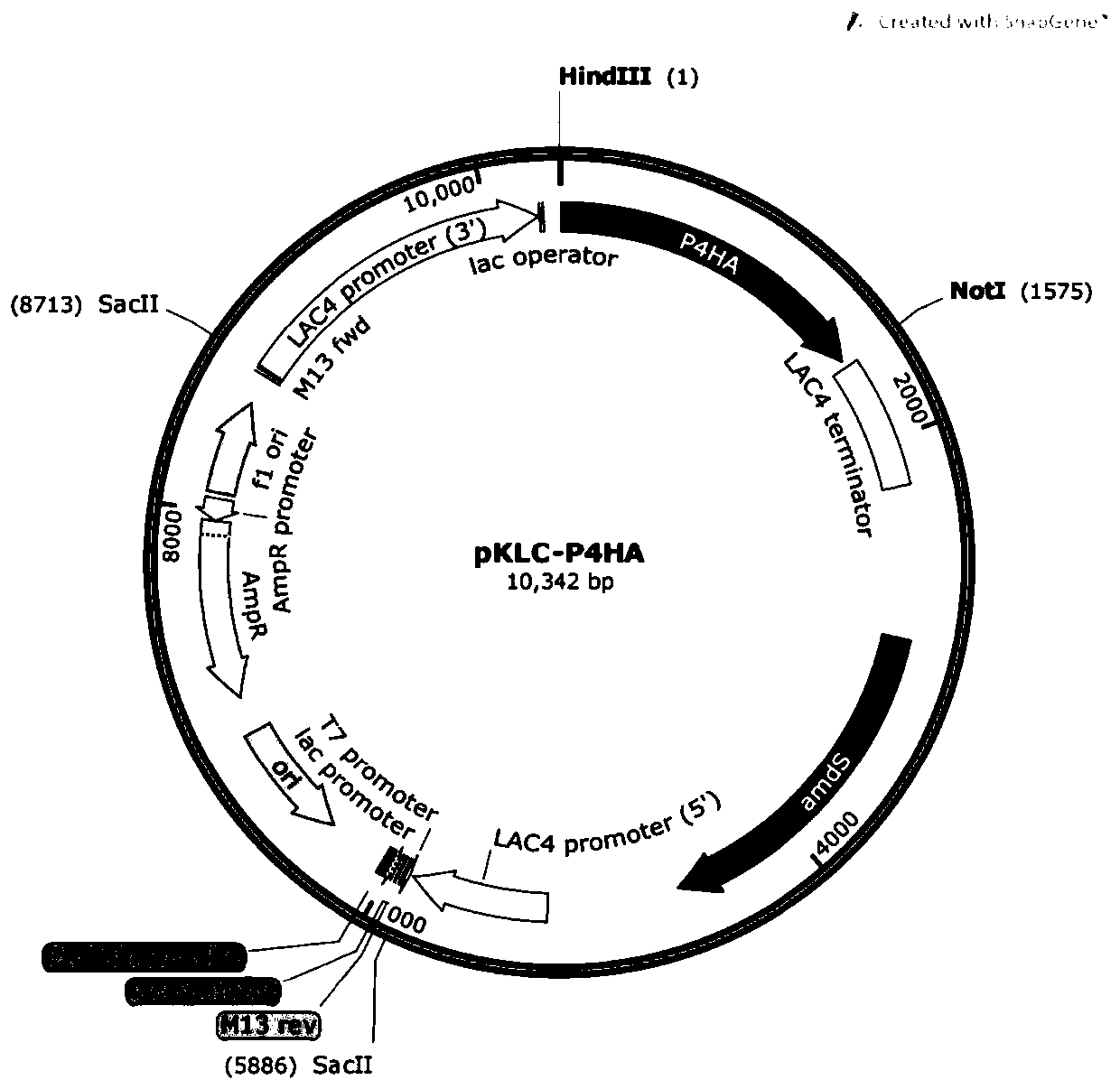
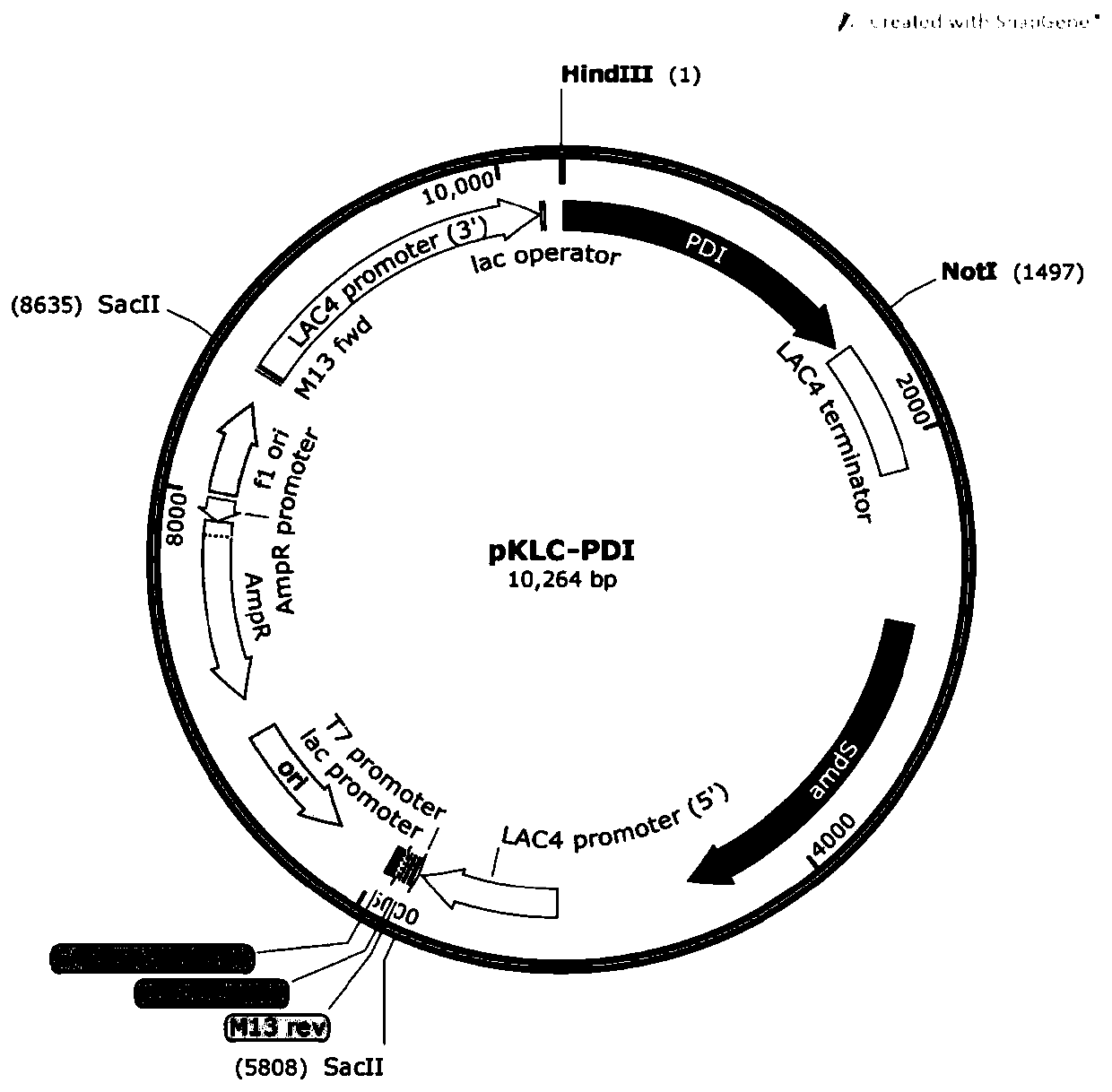
[0053] Such as figure 1 , 2 , 3, and 4, use restriction endonuclease SacII (U.S. NEB Company) to linearize plasmid A, plasmid B, plasmid C, and plasmid D, and construct an enzyme digestion reaction system as shown in Table 4:
[0054] Table 4 enzyme digestion reaction system
[0055]
[0056] The reaction conditions are 37°C, 30min. After the enzyme digestion reaction, use the gel recovery kit (Beijing Tiangen Biology) to recover the carrier fragment containing the target gene by tapping the rubber, and remove the resistance gene fragment on the carrier.
[0057] The recovered high-purity plasmid fragments were added to Kluyveromyces competent cells, incubated on ice for 2 minutes, and then electroporated (1.5kv-2.5kv). Then spread it on the YCB medium containing acetamide (5mM), culture it at 28°C for 2 days, and wait until a single colony grows on the plate, and obtain the preservation...
Embodiment 3
[0071] Embodiment 3: Hydroxyproline and hydroxylysine content determination
[0072] Hydroxyproline was carried out according to the spectrophotometer method in the Ministry of Agriculture industry standard "Determination of L-Hydroxyproline in Raw Milk" (NY / T 3130-2017).
[0073] Hydroxylysine was detected using a hydroxylysine ELISA detection kit (purchased from Shanghai Yiyan Biotechnology Co., Ltd.). The steps are as follows: Take 10 μL each of the diluted standard substance (included with the kit) or the fermentation supernatant (from Example 2), and add 40 μL of the diluent; do not add to the blank well. Add 100 μL of horseradish peroxidase (HRP)-labeled detection antibody to each well except the blank well, seal the reaction well with a sealing film, and incubate for 60 minutes in a 37°C water bath or incubator . Discard the liquid, pat dry on absorbent paper, fill each well with washing solution, let it stand for 1 min, shake off the washing solution, pat dry on abso...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com