Function and application of Carabin in treatment of fatty liver and diabetes mellitus type 2
A technology for diabetes and fatty liver, applied in the fields of medical preparations containing active ingredients, metabolic diseases, screening of compounds, etc., can solve the problems of lack of clinical trial support, etc., and achieve the effect of inhibiting fatty liver and type II diabetes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] [Example 1] Obtaining mouse fatty liver and type 2 diabetes model (diet induced obesity, DIO)
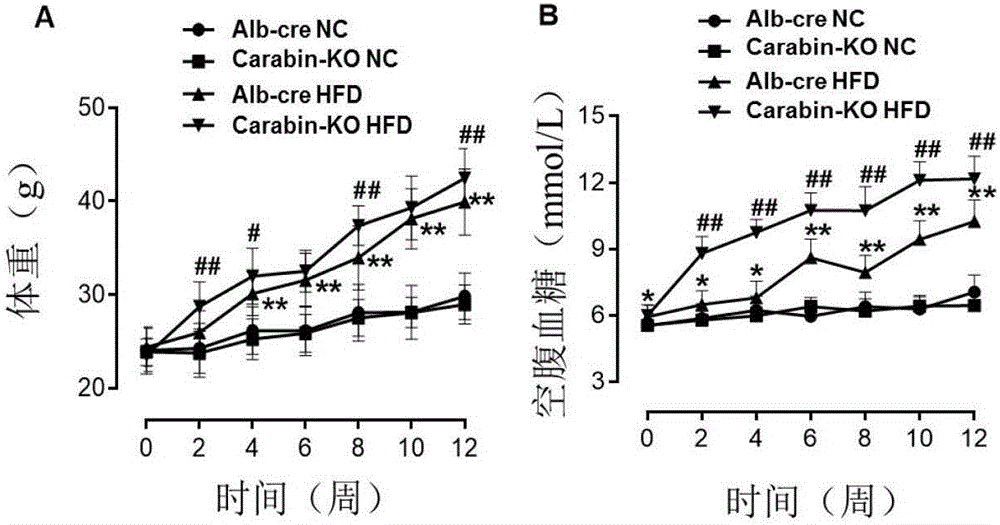
[0037] (1) Grouping of experimental animals: 8-week-old, male, Alb-cre mice and Carabin-KO mice were selected and given two special feeds, D12942 high-fat diet (High fat diet, HFD) and D12450B low-fat diet ( Normal chow, NC) feeding, that is, Alb-cre NC group, Carabin-KO NC group, Alb-cre HFD group, Carabin-KO HFD group, a total of 4 groups.
[0038] (2) The model induces the operation process through high-fat feed:
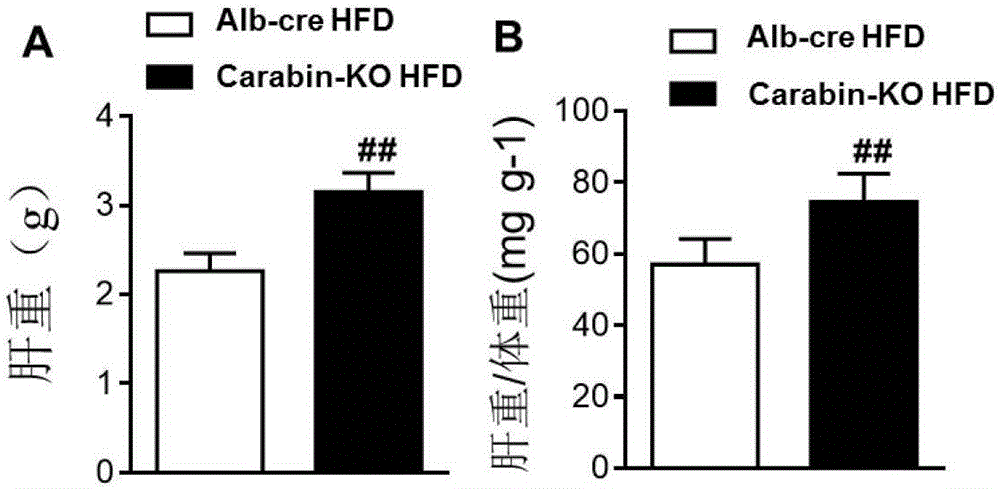
[0039] Alb-cre and Carabin-KO mice were used to establish DIO models, and phenotype correlation analysis was performed to clarify the role of Carabin gene in fatty liver and type Ⅱ diabetes. Eight-week-old, male, Alb-cre mice and Carabin-KO mice were selected and fed with two special diets, D12942 high fat diet (High fat diet, HFD) and D12450B low fat diet (Normalchow, NC), respectively, namely Alb-cre NC group, Carabin-KO NC group, Alb-cre HFD group, and Cara...
Embodiment 2
[0040] [Example 2] Determination of mouse body weight and blood sugar level
[0041] (1) Detection of fasting body weight of mice
[0042] ①Fasting: fast the mice to be tested at 8:00 am (without water), and start the experimental operation at 2:00 pm.
[0043] ② Weighing: Weigh at 0 week, 2 weeks, 4 weeks, 6 weeks, 8 weeks, 10 weeks, and 12 weeks respectively. In a small bucket, measure the weight and record the data.
[0044] (2) Fasting blood glucose level detection experiment
[0045] All the mice to be tested were fasted from 8:00 am to 2:00 pm (without water), that is, the experimental operation was started after 6 hours of fasting.
[0046] ① Blood glucose meter preparation: Check the battery of the blood glucose meter (Johnson & Johnson, ONETOUCH), press the right switch, put the test strip into the left slot correctly, the screen will display the code corresponding to the blood glucose test strip, and then display the dripping blood pattern, Indicates that the blo...
Embodiment 3
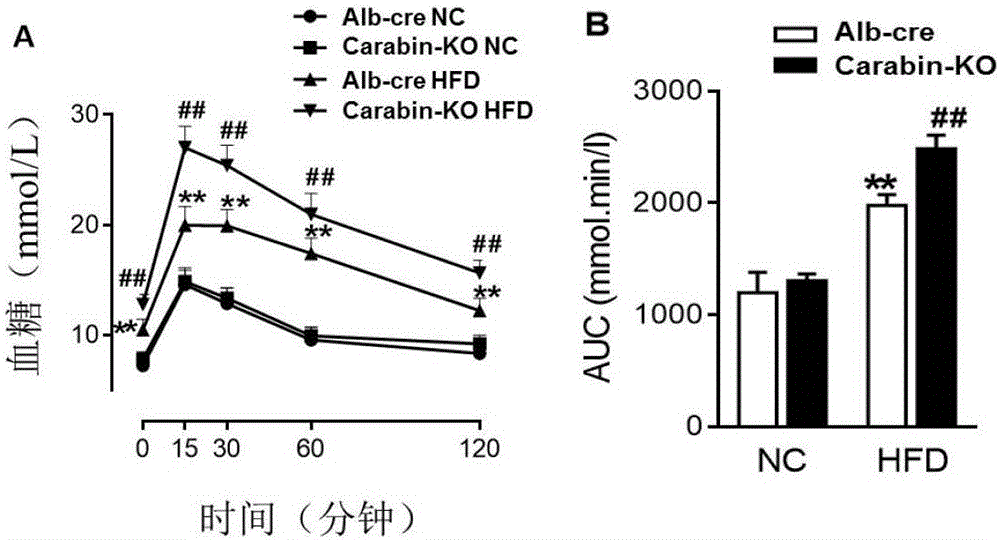
[0051][Example 3] Glucose tolerance test (intraperitoneal glucose tolerance test, IPGTT)
[0052] On the 10th week of the experiment, the intraperitoneal injection of glucose test (IPGTT) was performed to evaluate the glucose tolerance of the mice.
[0053] (1) Before measuring blood glucose, measure the fasting body weight of the mice, and calculate the injection volume of glucose based on 10 μL / g.
[0054] (2) First detect the fasting blood glucose level at 0 minutes before the glucose injection, and inject the glucose solution intraperitoneally quickly after the detection is completed.
[0055] (3) Operation method of intraperitoneal injection: ①Fix the mouse; grab the mouse, grab the tail of the mouse with the little finger and ring finger of the left hand, and grab the neck of the mouse with the other three fingers, so that the head of the mouse is downward, and the The abdomen of the mouse is fully exposed. ②Needle positioning and injection: Insert the needle from the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com