Composition containing NAD for preventing and treating obesity or impaired glucose tolerance
A technology for nicotinamide adenine and impaired glucose tolerance, which is applied in the fields of medical preparations containing active ingredients, drug combinations, food science, etc. and the effect of treating impaired glucose tolerance, inhibiting weight gain, and increasing exercise capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Experimental materials and methods
[0063] 1-1 Experimental animals
[0064] Mature male C57BL / 6 mice were purchased from Orientbio (Gyeonggi-do, Korea). Unless otherwise stated, mice were given ad libitum access to a standard diet (Agricultural Standard Purina, Seoul, Korea). To make a diet-induced obesity (DIO) model, mice were fed a high-fat diet (60% fat, Research Diet Co., New Brunswick, NJ) for 20 weeks. Animal feeding was carried out under the adjusted temperature (22±1° C.) and a 12-hour light-dark cycle (light conditions from 08:00 a.m to 8:00 p.m).
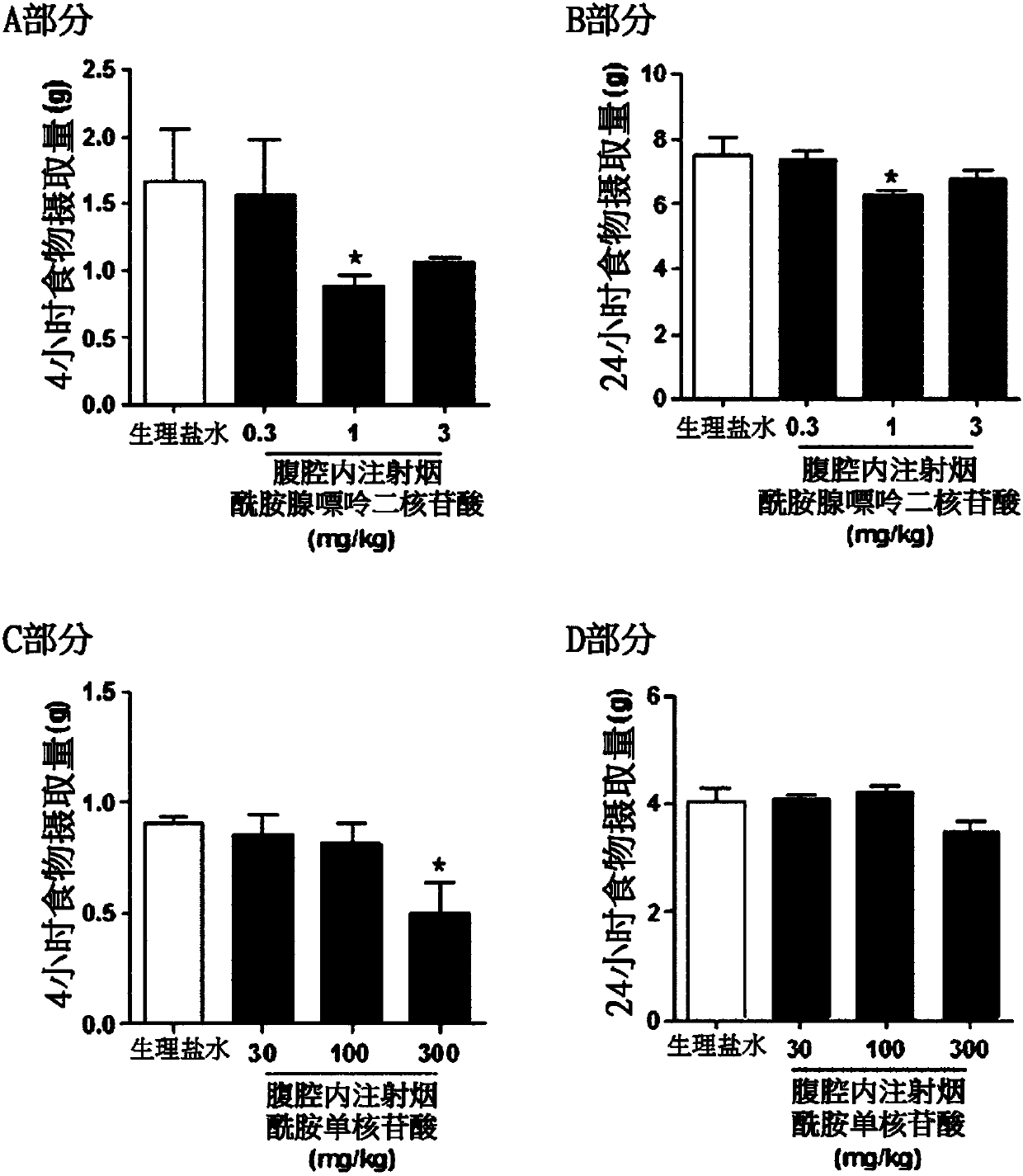
[0065] For the experiment of single administration of nicotinamide adenine dinucleotide and nicotinamide mononucleotide, nicotinamide adenine dinucleotide (purchased from Sigma, 0.3 mg / kg, 1mg / kg and 3mg / kg) or nicotinamide mononucleotide (purchased from Sigma, 30mg / kg, 100mg / kg and 300mg / kg) after fasting at night to 9-10 am . For long-term nicotinamide adenine dinucleotide therapy, nicotinamide adenine di...
Embodiment 2
[0080] Effects of administration of nicotinamide adenine dinucleotide on food intake and body weight in obese animal models
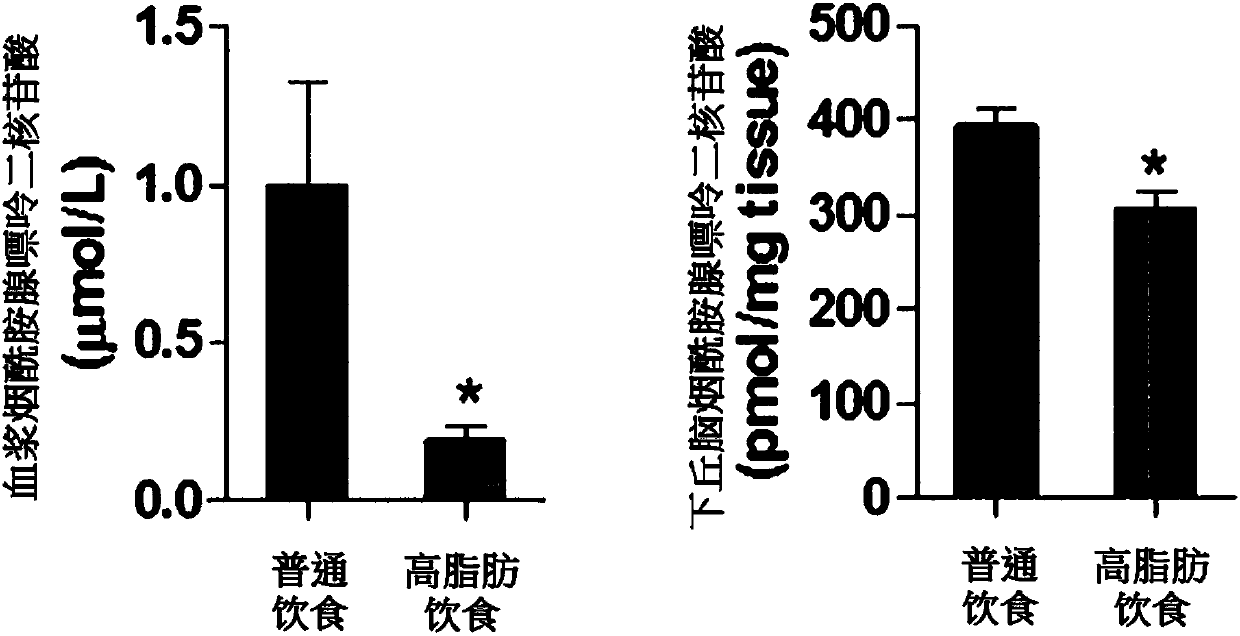
[0081] To determine the amount of nicotinamide adenine dinucleotide in animals that were induced to become obese by dietary intake, C57BL / 6 mice fed a high-fat diet (HFD) or normal diet (ND) were fed at week 20, Plasma and hypothalamus were collected for high performance liquid chromatography (HPLC, high performance liquid chromatography) for the determination of nicotinamide adenine dinucleotide ( figure 1 ). Compared with the mice fed the normal diet, the mice fed the high-fat diet for 20 weeks showed a significant decrease in the amount of nicotinamide adenine dinucleotide in both plasma and hypothalamus (P<0.05).
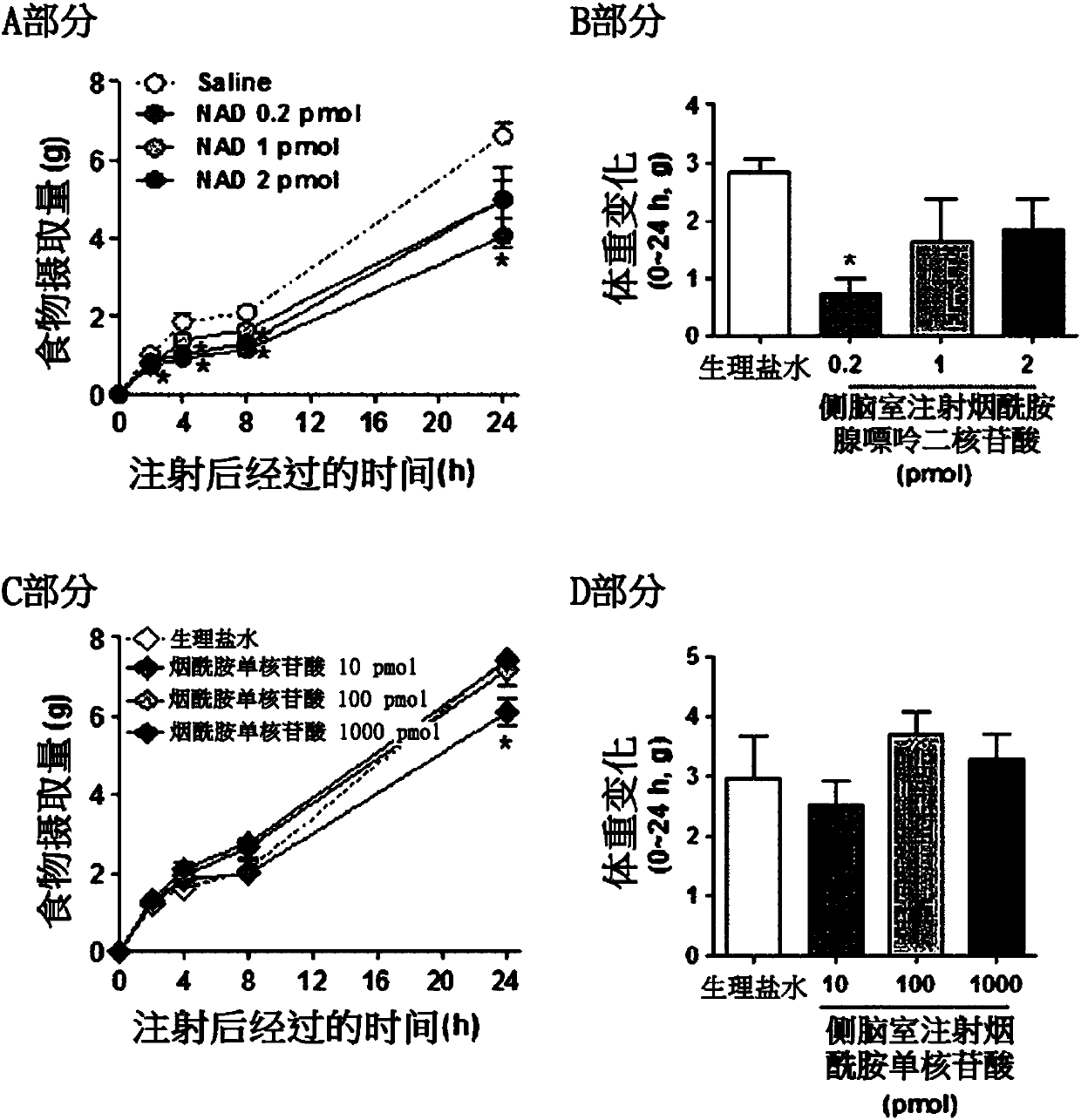
[0082] In order to examine the effect of administration of nicotinamide adenine dinucleotide on the food intake of obese animal models, nicotinamide adenine dinucleotide was intracerebroventricularly or intraperitoneally administered, an...
Embodiment 3
[0089] Effects of administration of nicotinamide adenine dinucleotide on physical activity in an animal model of obesity
[0090] In order to test the effect of chronic intraperitoneal injection of nicotinamide adenine dinucleotide (0.3mg / kg / day) on the exercise of obese animal models, these mice were divided into the group of intraperitoneal injection of normal saline ingestion of normal diet, ingestion of Three groups, the high-fat diet intraperitoneal injection of normal saline group and the high-fat diet intraperitoneal injection of nicotinamide adenine dinucleotide group, were evaluated for exercise performance. The amount of physical activity of the normal control group injected with normal saline and ingested a normal diet was significantly higher at night (equivalent to the daytime of humans) than in the daytime, and thus a significant diurnal cycle rhythm of the amount of physical activity was observed. On the contrary, the obese group ingesting a high-fat diet admini...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com