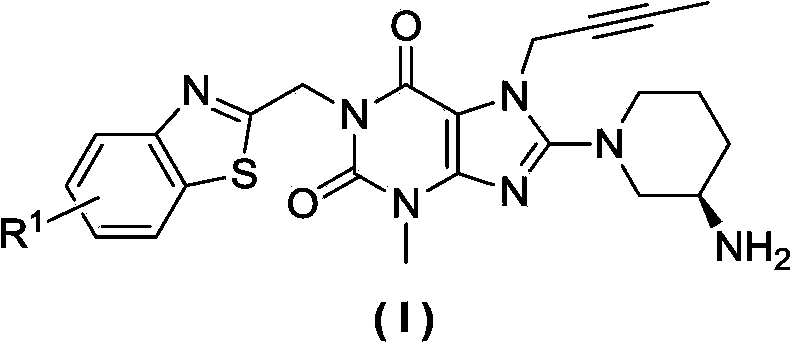
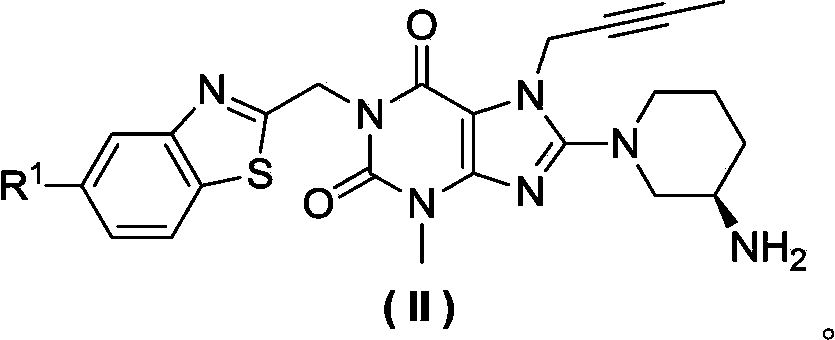
Xanthine derivative
A compound, pharmaceutical technology, applied in the field of dipeptidyl peptidase IV inhibitor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
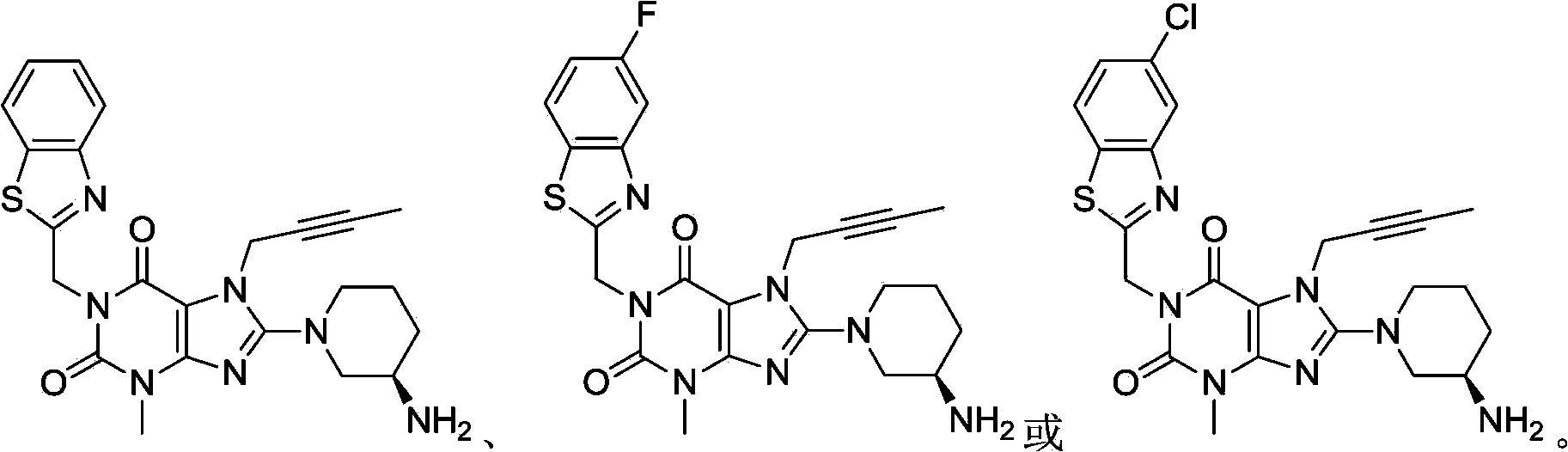
[0031] 1-[(5-fluoro-1,3-benzothiazol-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[(R)-3 Preparation of -amino-piperidin-1-yl]-xanthine
[0032]
[0033] Preparation of the first step 3-methyl-7-(2-butyn-1-yl)-8-bromo-xanthine
[0034] Using known methods, 8-bromo-3-methylxanthine (5g, 20.4mmol) was dissolved in N,N-dimethylformamide (30ml), N,N diisopropylethylamine (2.633 g, 20.4mmol), 1-bromo-2-butyne (2.714g, 20.4mmol), react overnight at room temperature, follow the reaction process by thin-layer chromatography, after the reaction is complete, pour the reaction solution into water, filter with suction, and wash the solid with water for 3 times and dried to obtain 3-methyl-7-(2-butyn-1-yl)-8-bromo-xanthine 1a (5.15 g, light yellow solid), yield: 85%.
[0035] MS m / z(ES):297,299[M+1]
[0036] The second step 1-[(5-fluoro-1,3-benzothiazol-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-bromo-yellow Preparation of purine
[0037] 3-Methyl-7-(2-butyn-1-yl)-8-bromo-xanthine 1a (15...
Embodiment 2
[0047] 1-[(1,3-Benzothiazol-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[(R)-3-amino-piper Preparation of pyridin-1-yl]-xanthine
[0048]
[0049]
[0050] The first step is the same as the first step in implementation example 1;
[0051] The second step is the preparation of 1-[(1,3-benzothiazol-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-bromo-xanthine
[0052] 3-Methyl-7-(2-butyn-1-yl)-8-bromo-xanthine 1a (327mg, 1mmol) was dissolved in N,N-dimethylformamide (5ml) by a known method Add potassium carbonate (221mg, 1.6mmol), 2-bromomethyl-1,3-benzothiazole (228mg, 1mmol), react overnight at room temperature, track the reaction progress by thin-layer chromatography, after the reaction is complete, pour the reaction solution into water, filtered with suction, washed the solid with water, and dried to obtain 1-[(1,3-benzothiazol-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)- 8-Bromo-xanthine 2b (400 mg, yellow solid), yield: 90%.
[0053] MS m / z(ES):444,446[M+1]
[0054] The third...
Embodiment 3
[0062] 1-[(5-chloro-1,3-benzothiazol-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[(R)-3 Preparation of -amino-piperidin-1-yl]-xanthine
[0063]
[0064] The first step is the same as the first step in implementation example 1;
[0065] The second step 1-[(5-chloro-1,3-benzothiazol-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-bromo-yellow Preparation of purine
[0066] Using a known method, 3-methyl-7-(2-butyn-1-yl)-8-bromo-xanthine 1a (297mg, 1mmol) was dissolved in N,N-dimethylformamide (8ml) , add 2-bromomethyl-5-chloro-1,3-benzothiazole (263mg, 1mmol), potassium carbonate (213mg, 1.5mmol), react at room temperature overnight, after TLC tracking the reaction is complete, pour into water , filtered with suction, washed with water, and dried to give 1-[(5-chloro-1,3-benzothiazol-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl) -8-Bromo-xanthine 3b (460 mg, pale yellow solid), yield: 96%.
[0067] MS m / z(ES):478,480[M+1]
[0068] The third step 1-[(5-chloro-1,3-benzothiazol-2-yl)methyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com