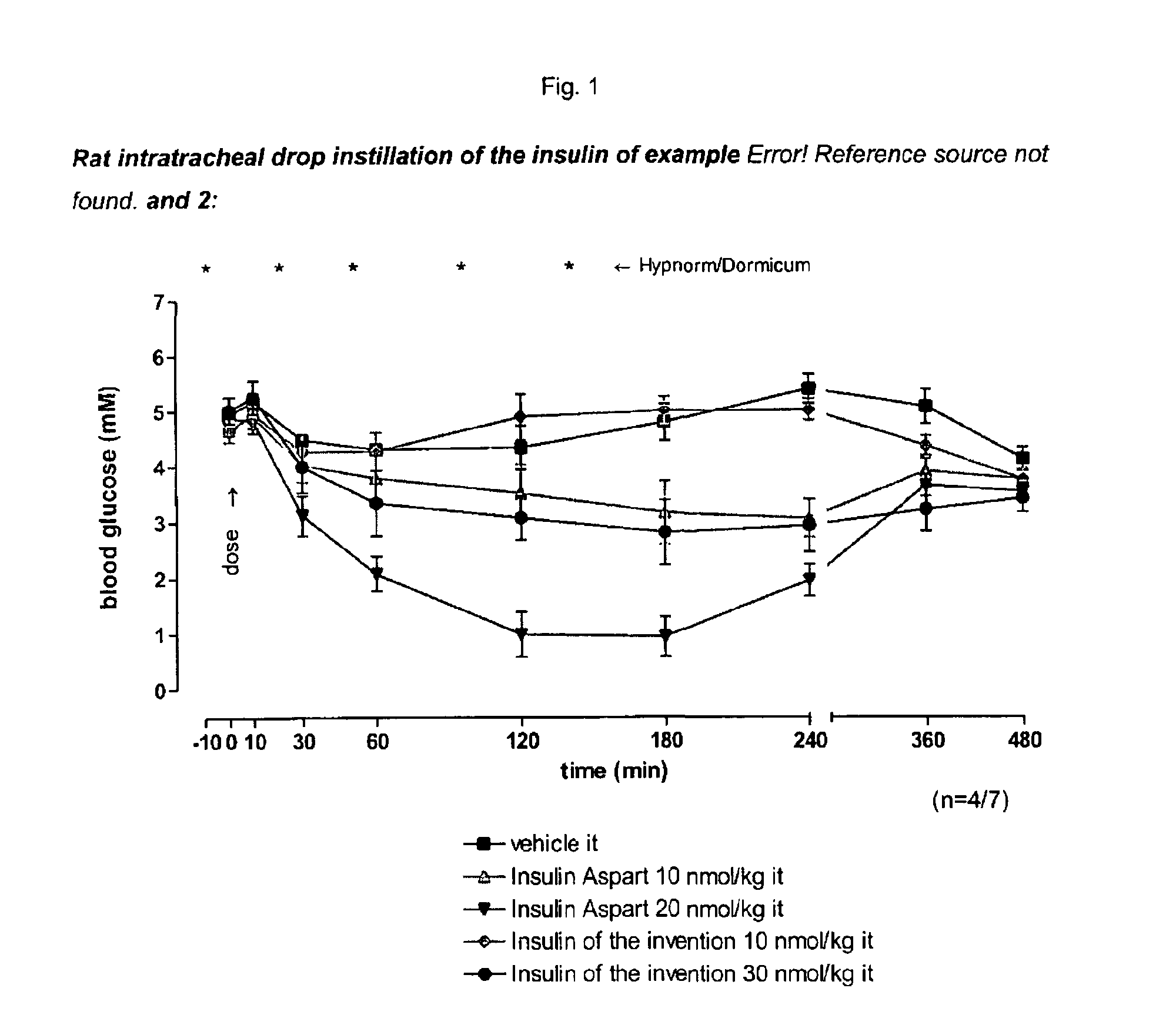
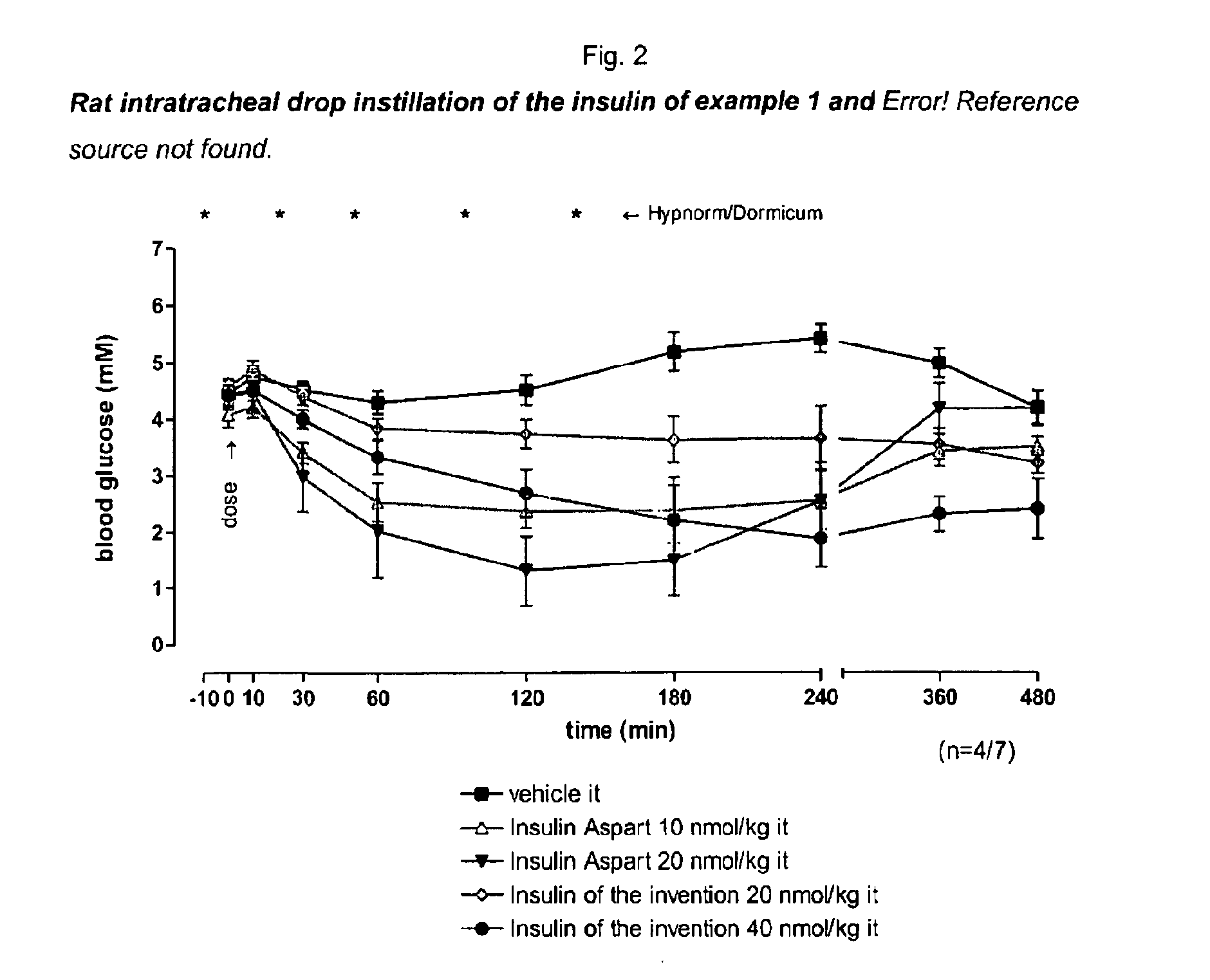
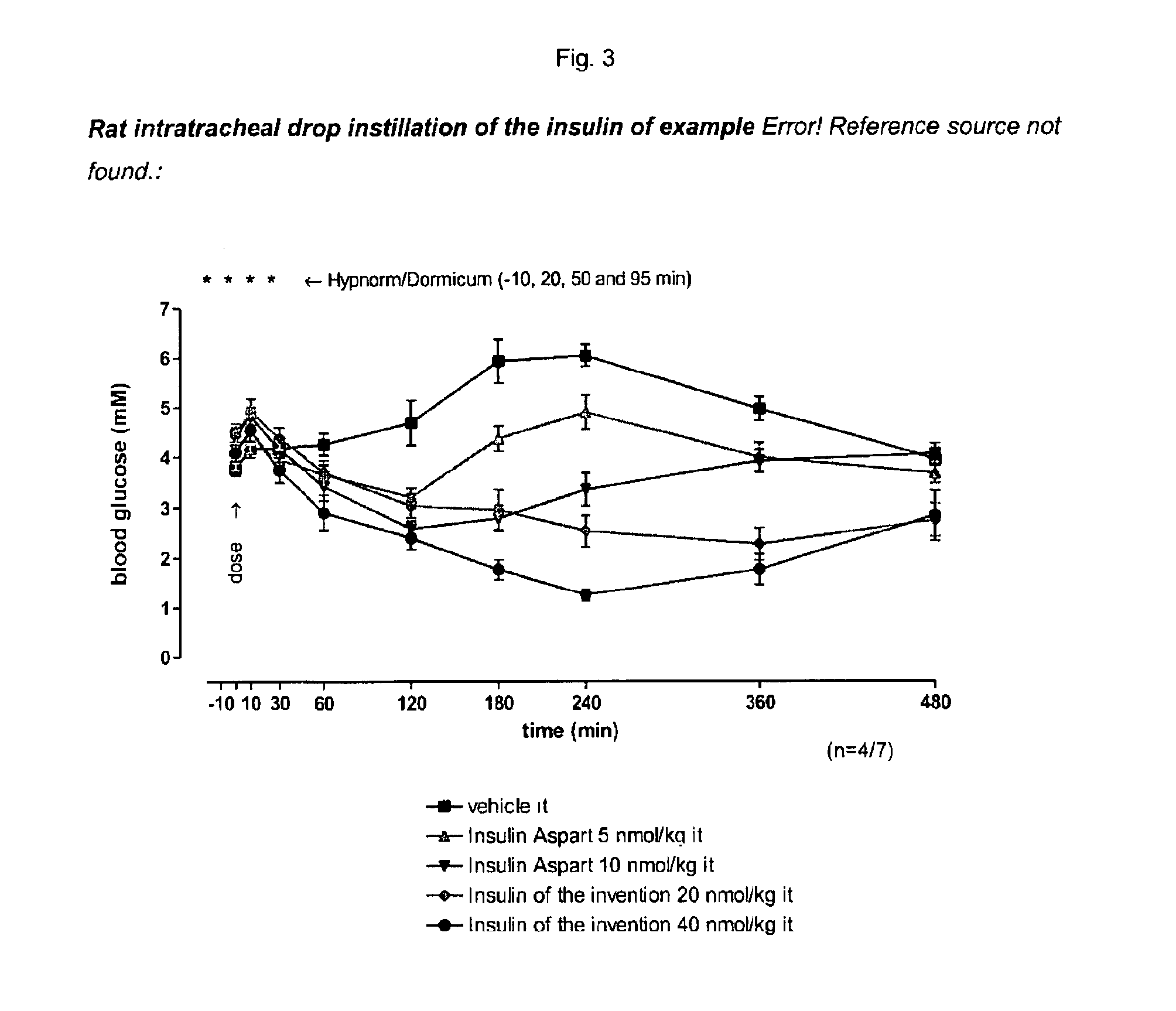
Pegylated, Extended Insulins
a technology of pegylated and extended insulin, applied in the direction of peptide/protein ingredients, drug compositions, metabolic disorders, etc., can solve the problems of ineffective insulin absorption, psychological and physical pain of therapy type, and many diabetics are unwilling to undertake intensive therapy, etc., to achieve the effect of sufficient chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
General Procedure (A)
[0238]A22K(Nε-mPEG2000-Propionyl), B29R, desB30 Human Insulin
Step 1: Preparation and Purification of the Insulin Precursor LysA22 ArqB29 B29R desB30 B′A
[0239]The insulin precursor A22K, B29R, desB30, B′A single chain insulin can be purified as described in the purification steps A to C below.
Purification step A: Capture
[0240]In step A, 10.75 litres of cleared culture media is diluted by addition of 4.5 litres of 99% ethanol, to give a total volume of 15.25 litres containing 30 vol % ethanol (conductivity 2.7 mS / cm, pH=3.4). A 300 ml SP Big Beads Sepharose column (100-300 μm, Amersham Biosciences) was equilibrated with 1 litre of 0.1 M citric acid pH 3.5 (flow app. 20 ml / min), before loading the 15.25 litres of prepared culture media over night (flow app. 10 ml / min). After loading the column was again washed with 1 litre of 0.1 M citric acid pH 3.5 followed by 1 liter of 40 vol % ethanol (flow app. 20 ml / min). The bound insulin precursor A22K, B29R, desB30, B′A s...
example 2
General Procedure (B)
[0245]A22K(NεmPEG2000-propionyl), B29R, desB30 Human Insulin
[0246]A22K, B29R, desB30 human insulin (125 mg) was dissolved in 0.1 M Na2CO3 (2.8 ml). mPEG-SPA 2000 (50 mg) dissolved in acetonitrile (1.25 ml) was added. pH was adjusted from 10.2 to 10.4 with 0.1 N NaOH. After 50 min more mPEG-SPA 2000 (25 mg) dissolved in acetonitrile (1.25 ml) was added. After slow stirring for 80 min, water (4.5 ml) was added and pH was adjusted to 5 with 1 N HCl. The mixture was lyophilized. The title compound was obtained by preparative HPLC purification. Column: C4, 2 cm. A-Buffer: 0.1% TFA in MiliQ Water; B-buffer: 0.1% TFA in acetonitrile. Gradient 30-65% B over 30 min. Yield 43 mg.
[0247]MALDI-MS (matrix: sinapinic acid); m / z: 8114.
example 3
General Procedure (B)
[0248]A22K(NεmPEG750-Propionyl, B29R, desB30 Human Insulin
[0249]MALDI-MS (matrix: sinapinic acid); m / z: 5862.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com