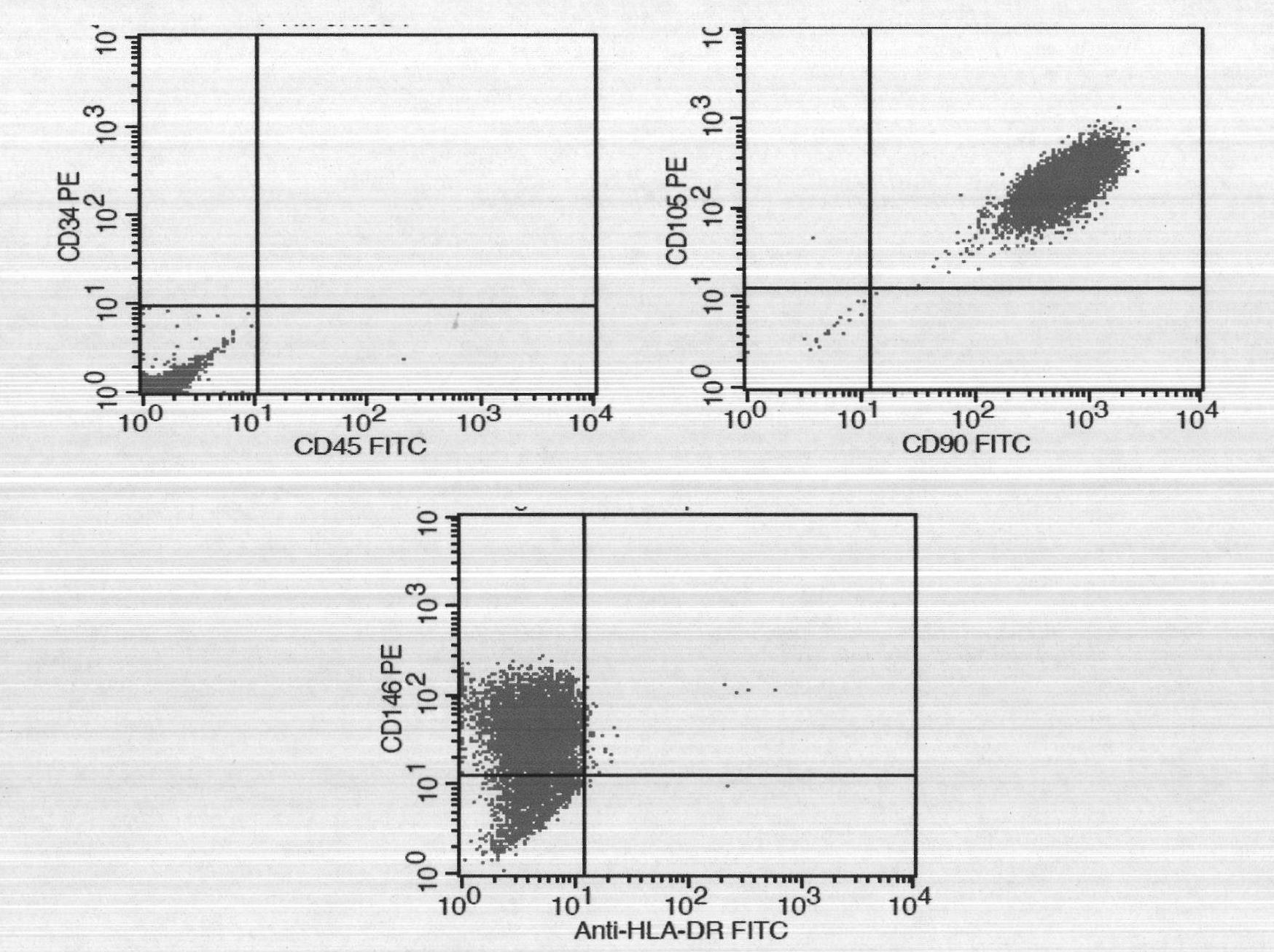
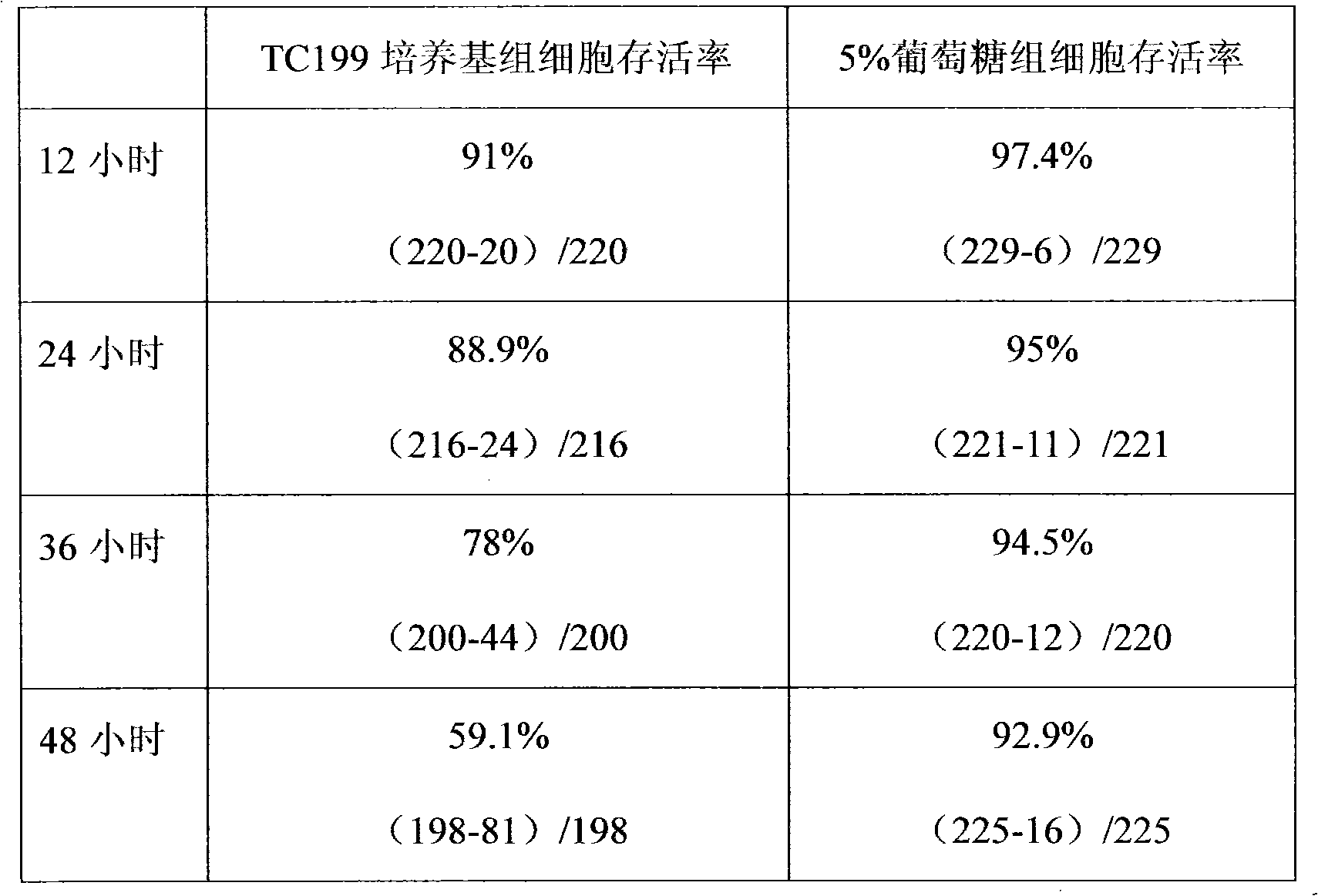
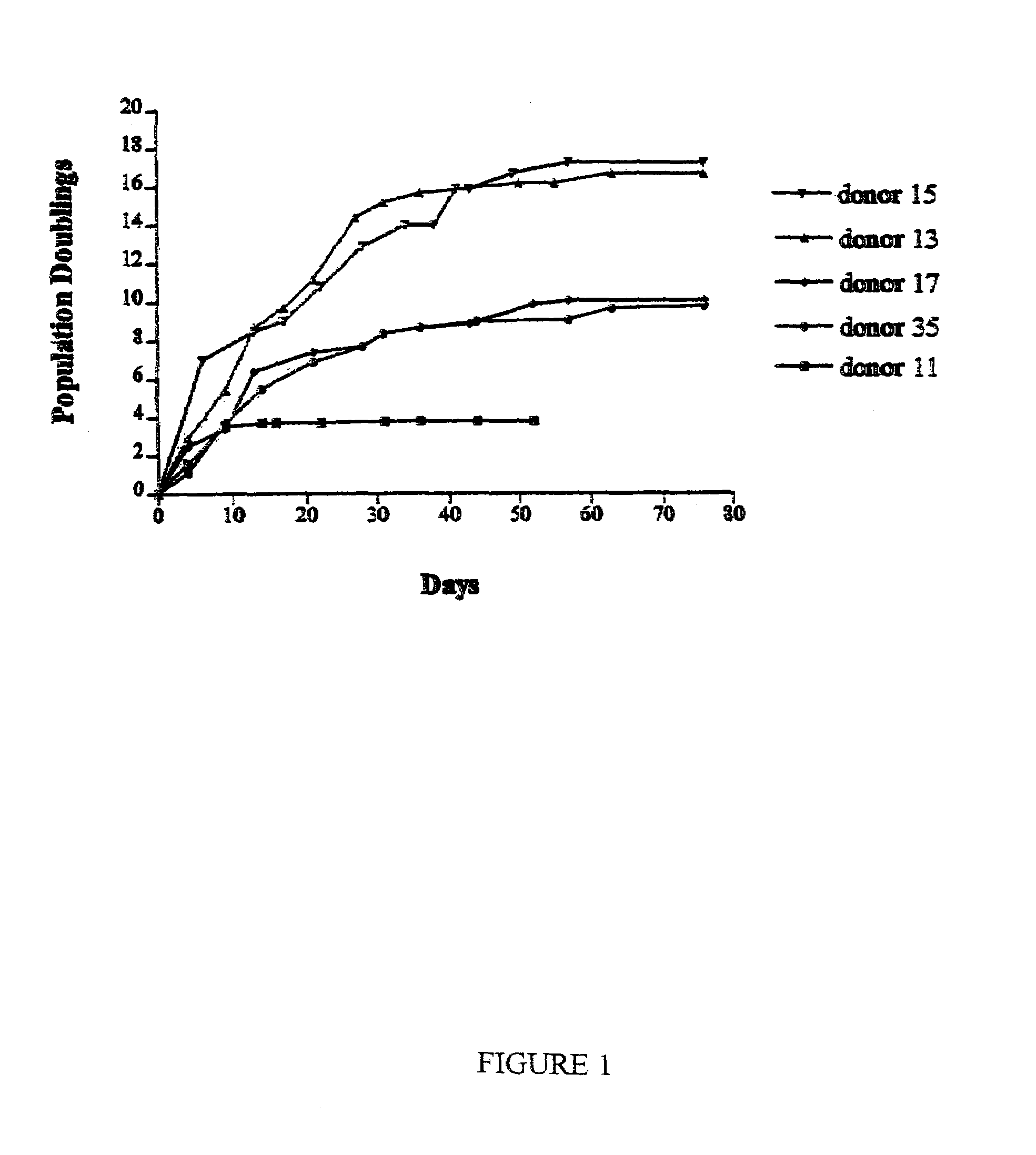
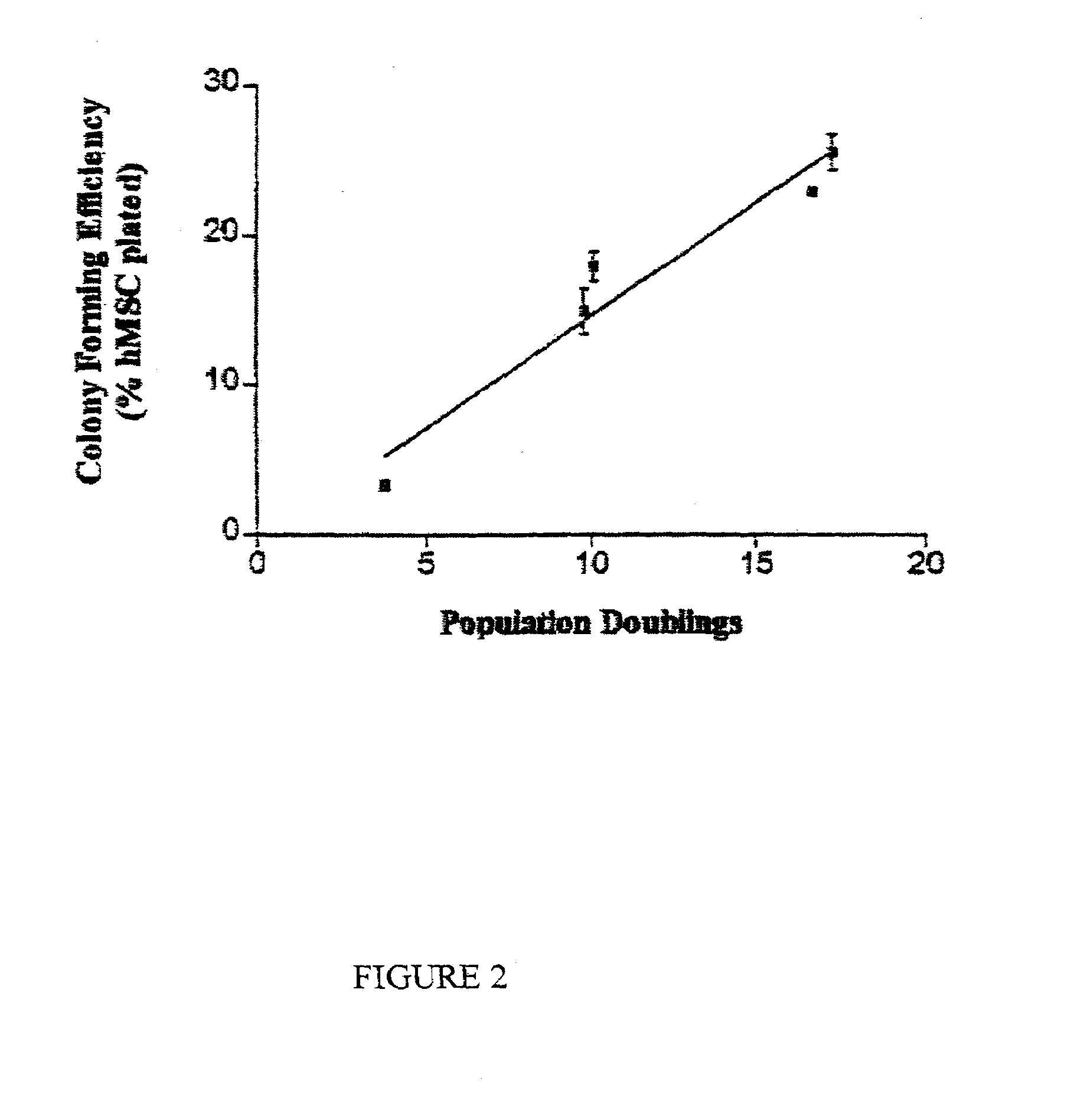
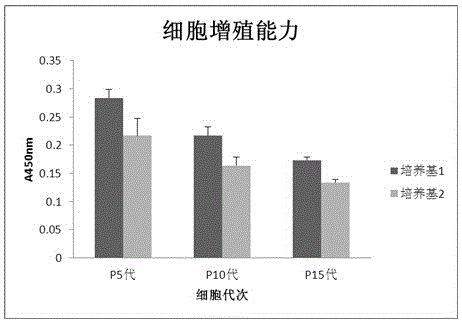
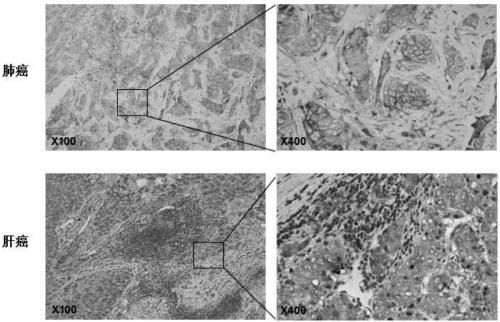
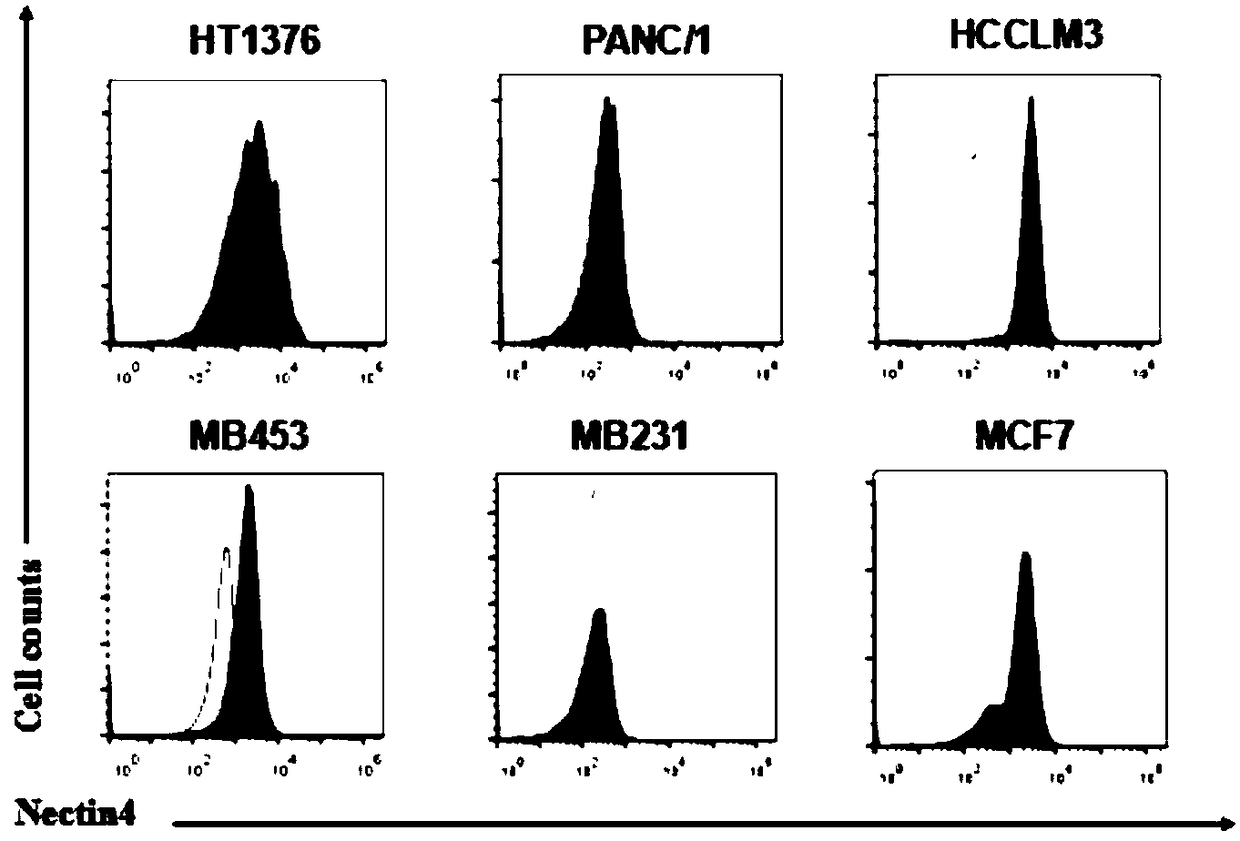
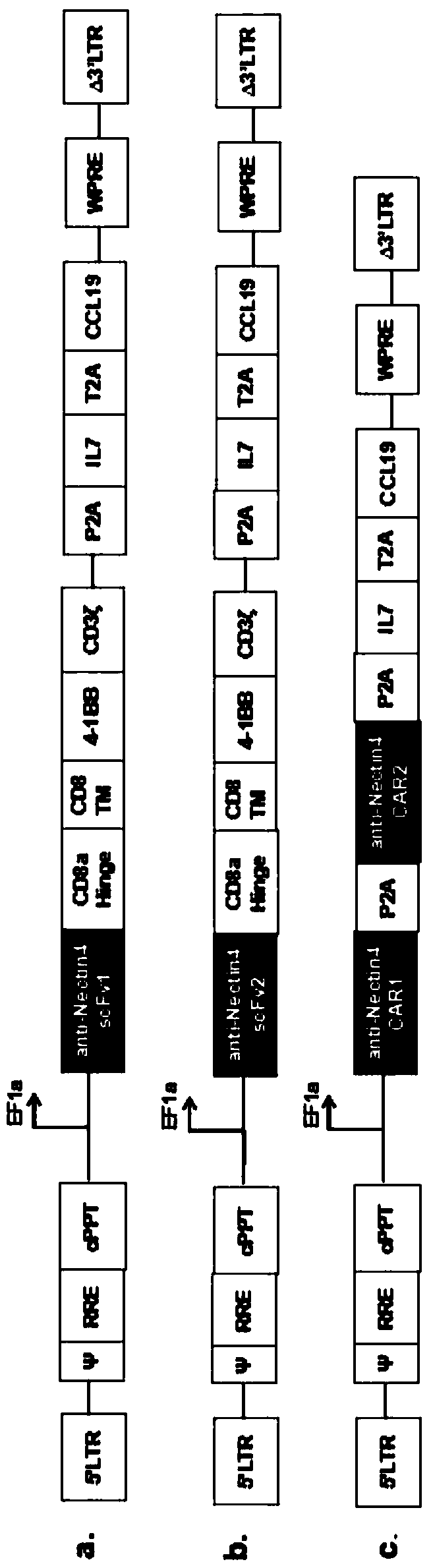
Patents
Literature
200 results about "Proliferative capacity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Isolation And Use Of Solid Tumor Stem Cells
InactiveUS20080178305A1Reduce spreadIncreased proliferationMaterial nanotechnologyMicrobiological testing/measurementAbnormal tissue growthMammary gland structure
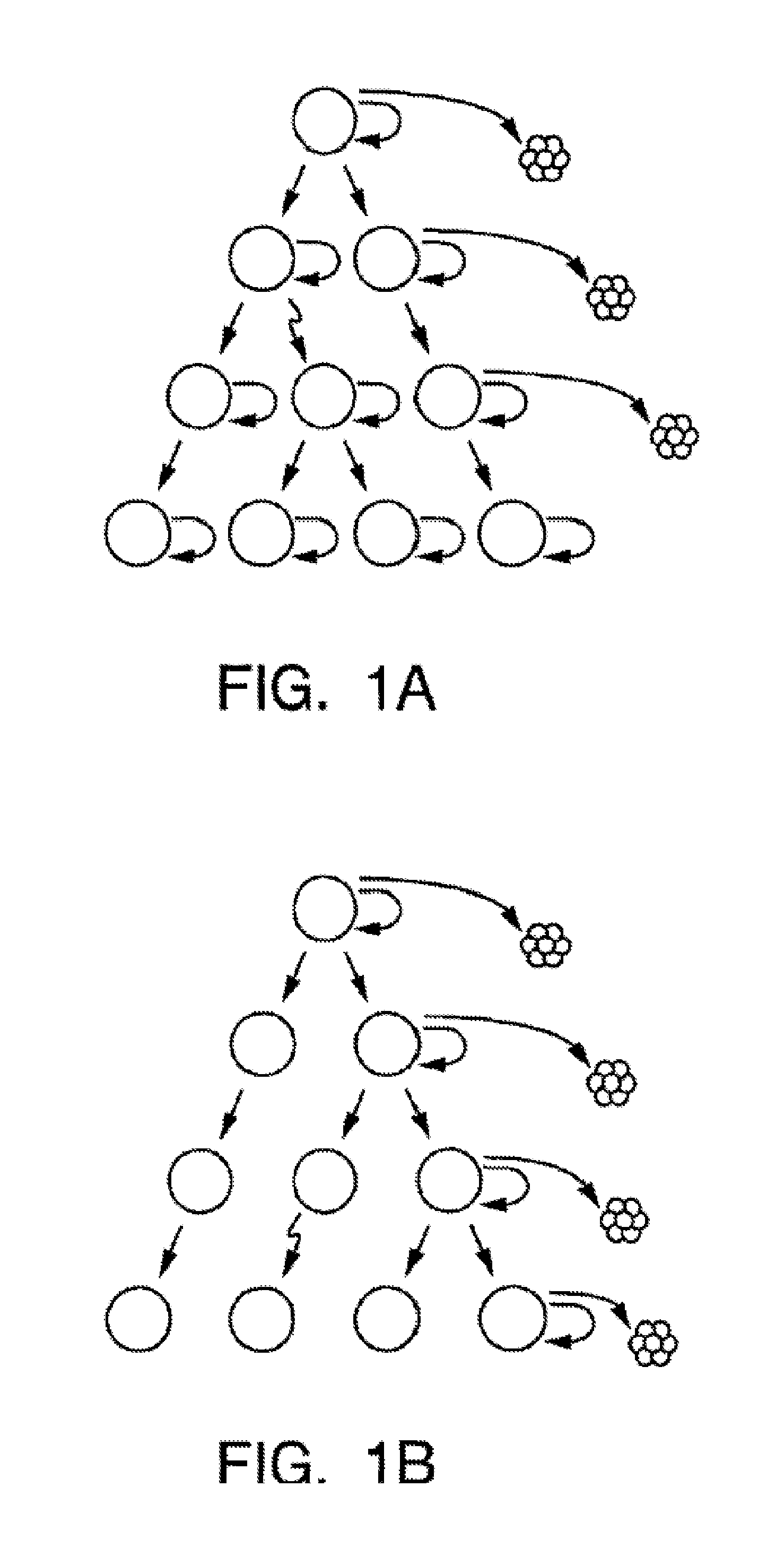
A small percentage of cells within an established solid tumor have the properties of stem cells. These solid tumor stem cells give rise both to more tumor stem cells and to the majority of cells in the tumor that have lost the capacity for extensive proliferation and the ability to give rise to new tumors. Thus, solid tumor heterogeneity reflects the presence of tumor cell progeny arising from a solid tumor stem cell. We have developed a xenograft model in which we have been able to establish tumors from primary tumors via injection of tumor cells in the mammary gland of severely immunodeficient mice. These xenograft assay have allowed us to do biological and molecular assays to characterize clonogenic solid tumor stem cells. We have also developed evidence that strongly implicates the Notch pathway, especially Notch 4, as playing a central pathway in carcinogenesis.
Owner:ONCOMED PHARMA +1
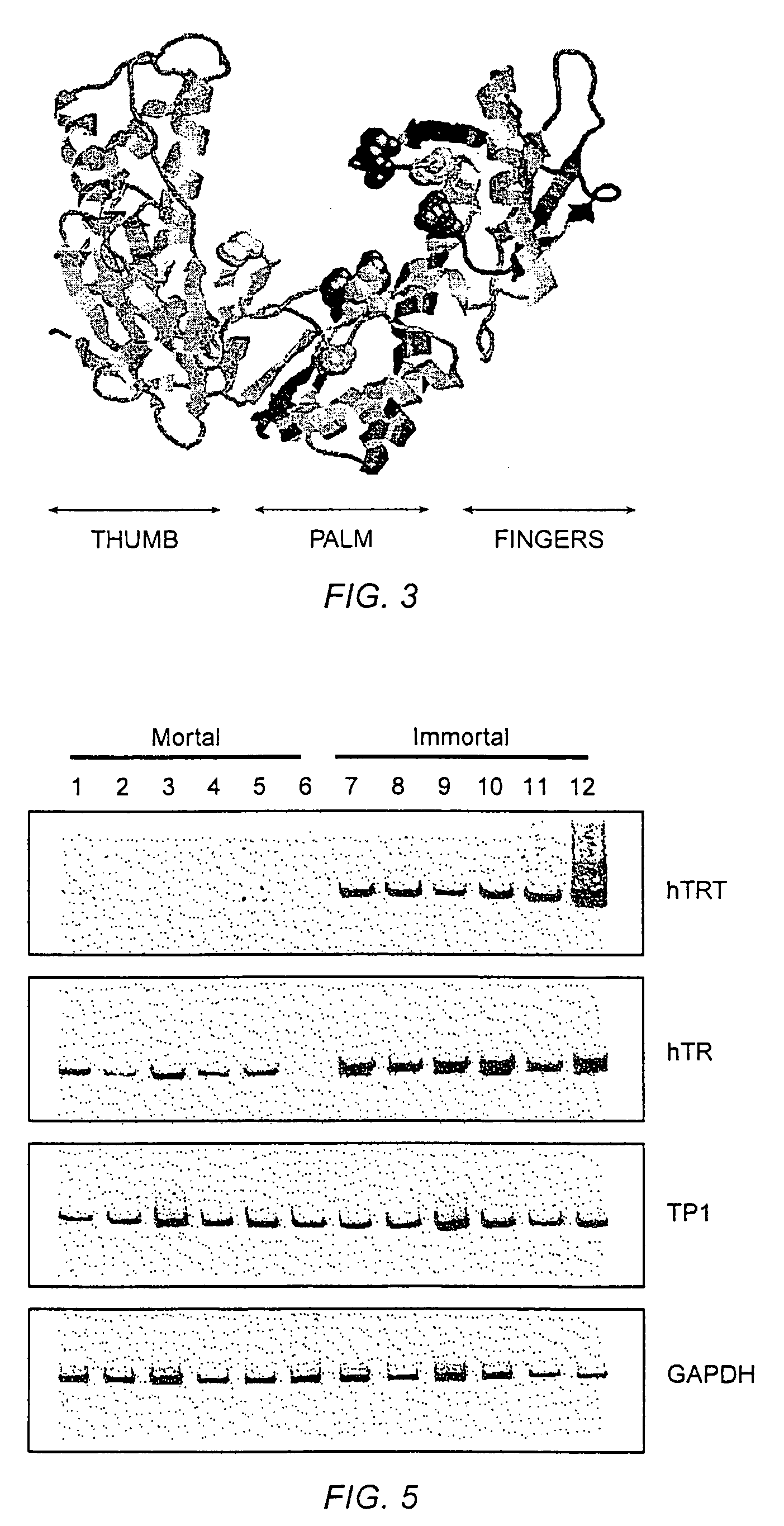
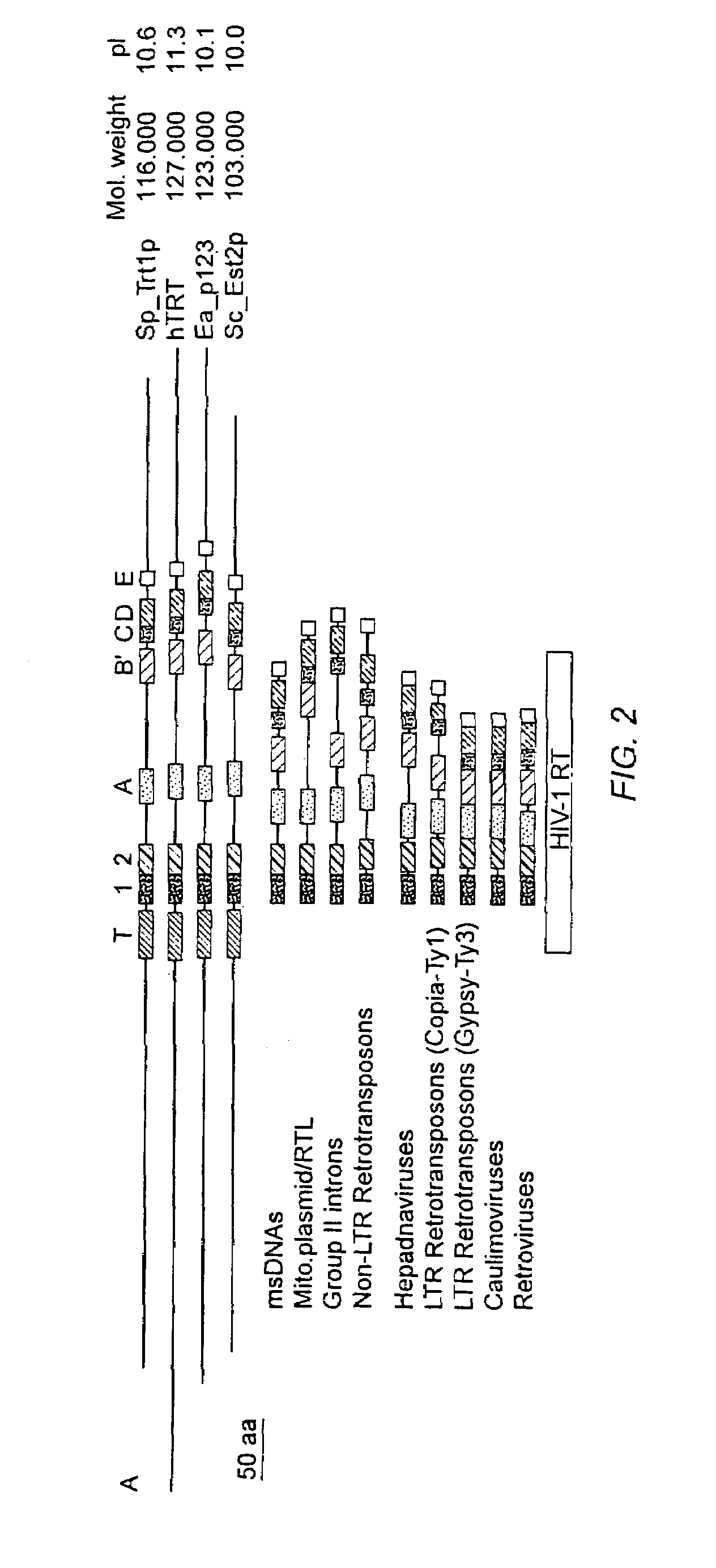
Nucleic acids encoding human telomerase reverse transcriptase and related homologs
InactiveUS7262288B1Improve proliferative abilityOrganic active ingredientsFungiBiological bodyReverse transcriptase
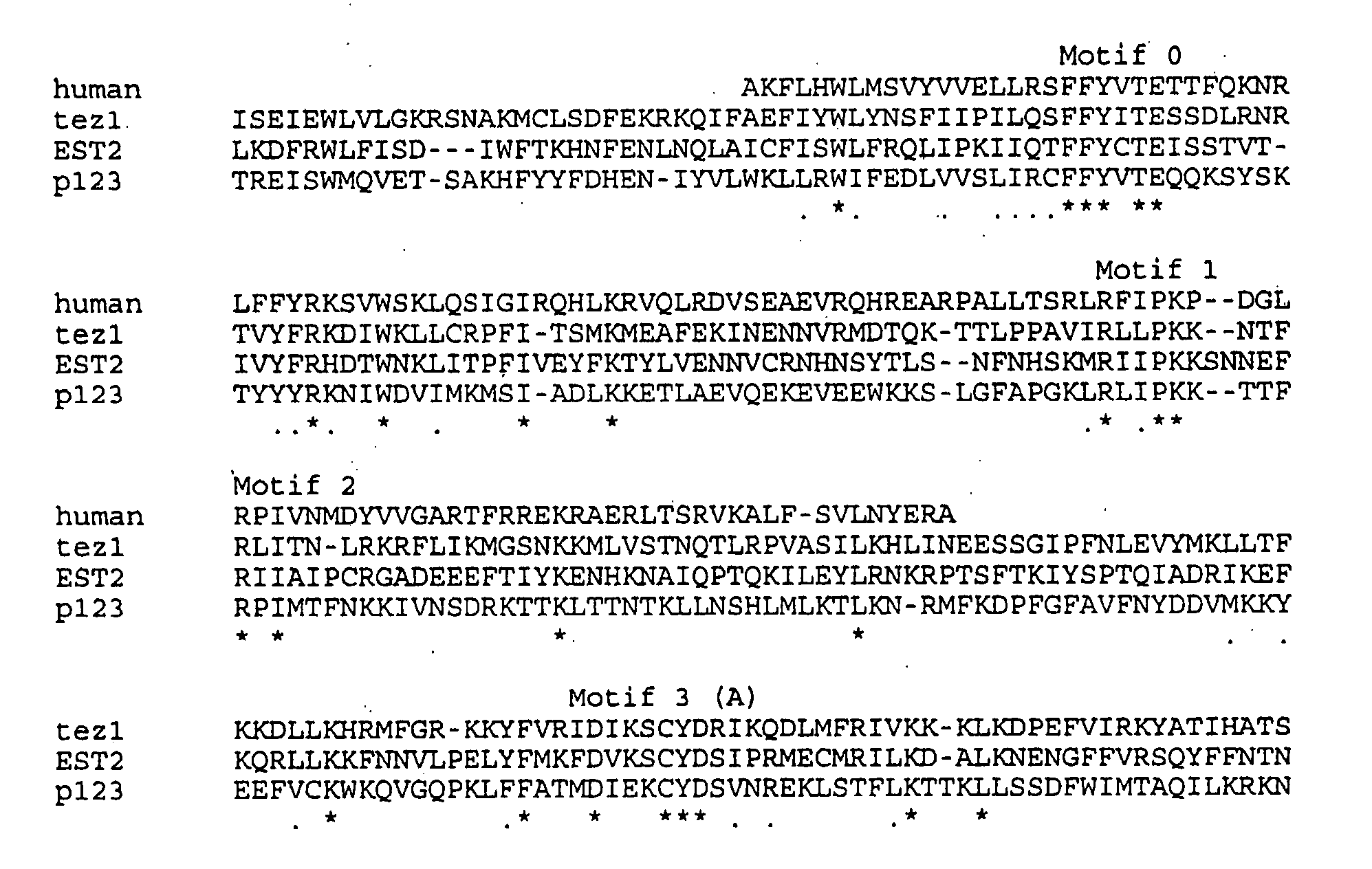
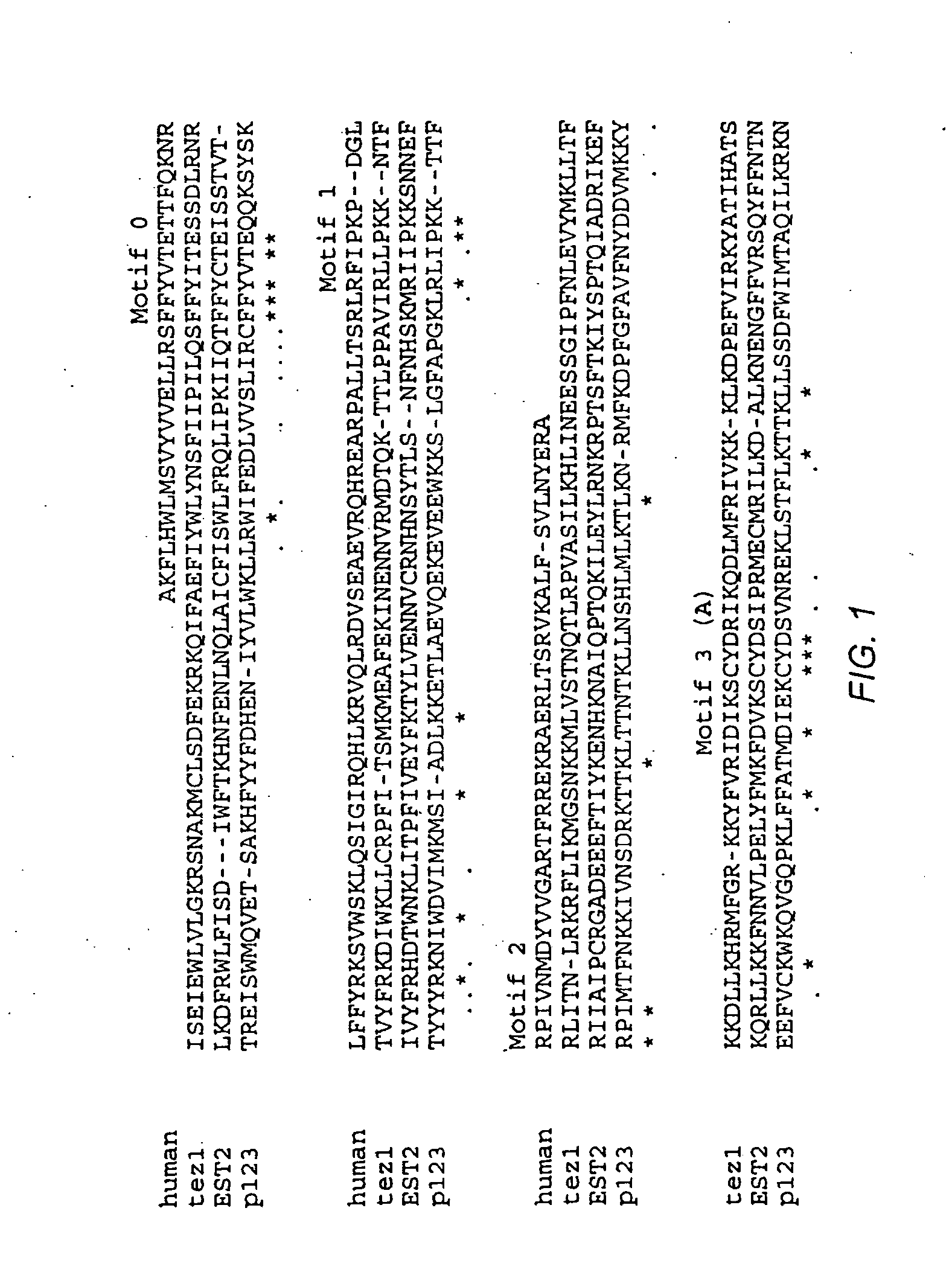
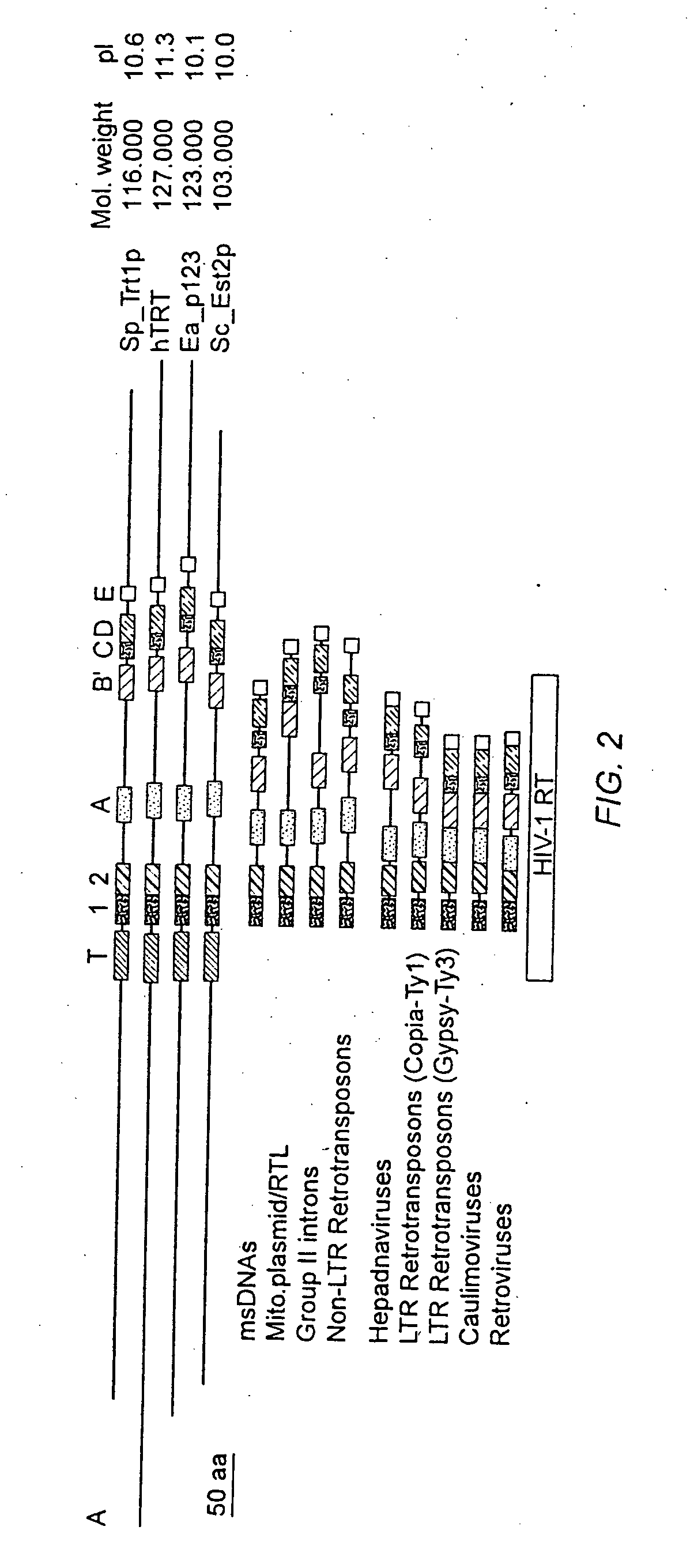
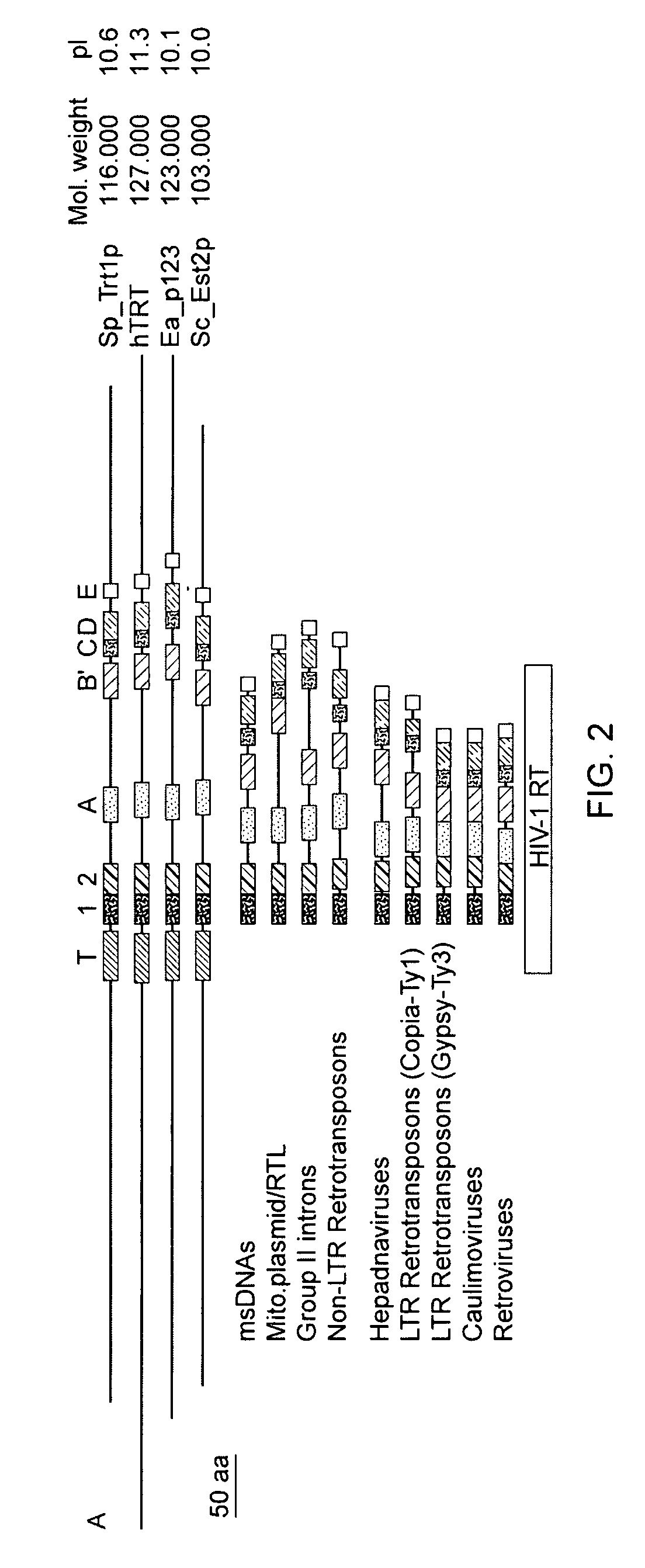
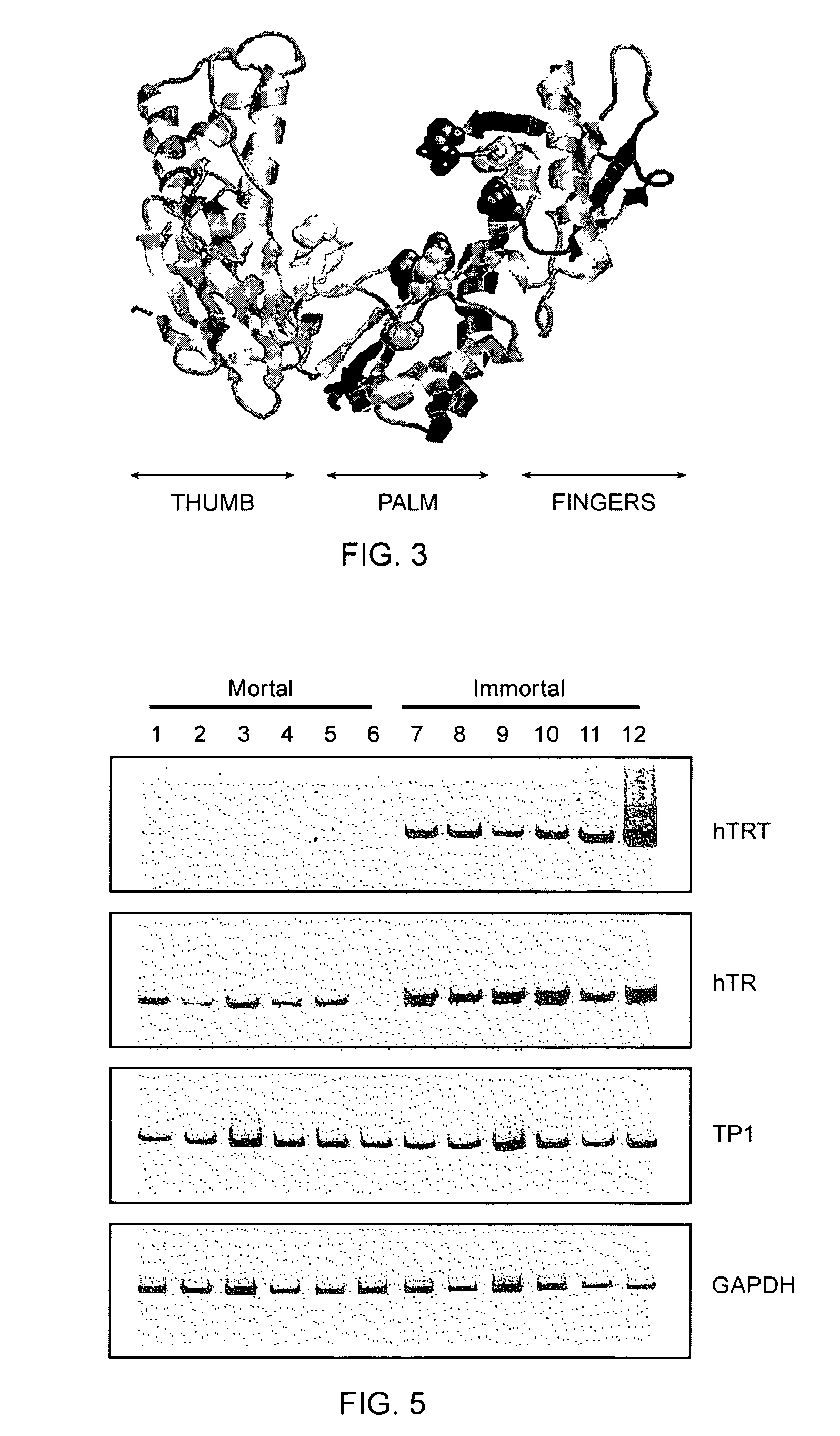
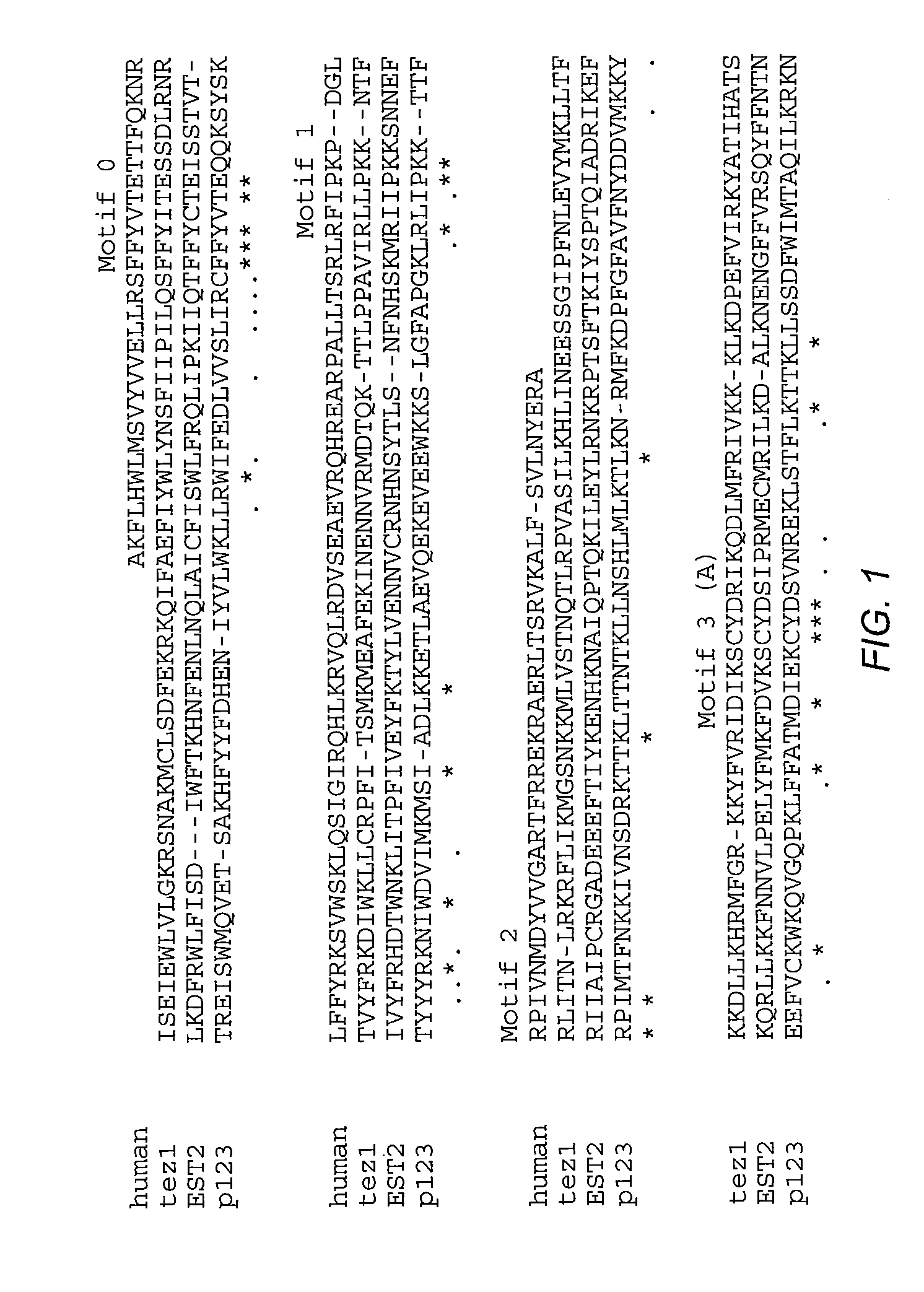
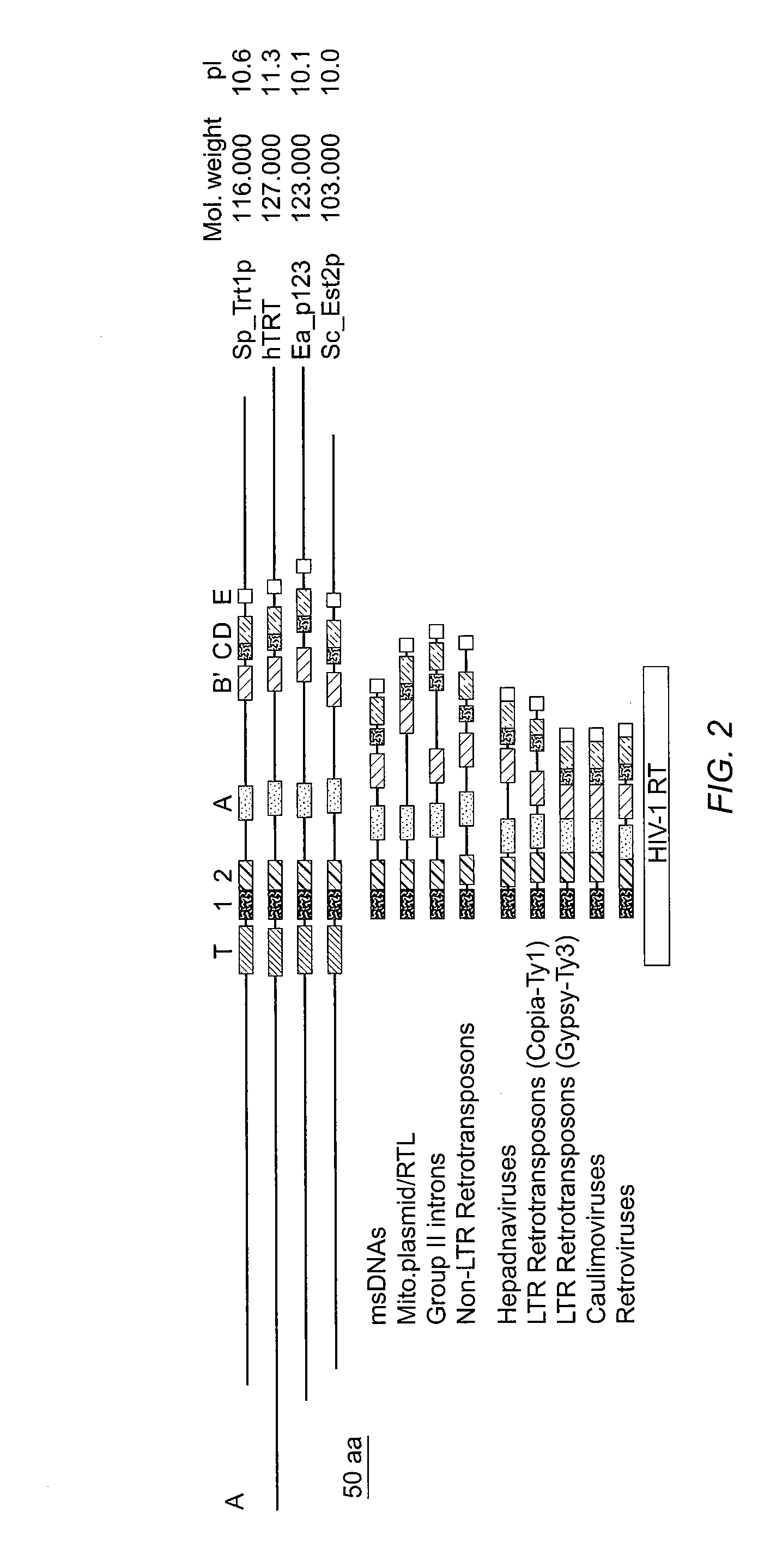
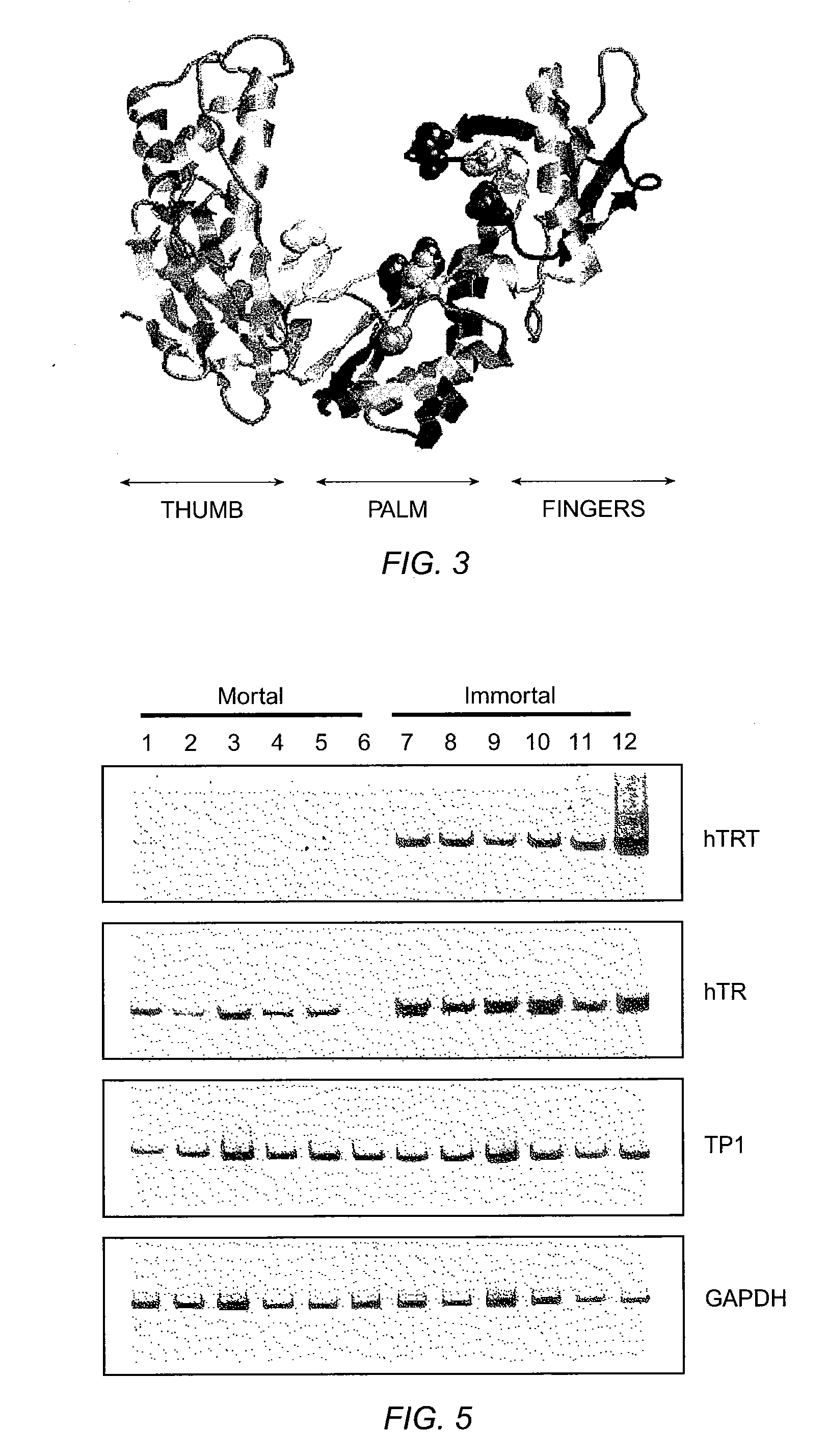
The invention provides compositions and methods related to human telomerase reverse transcriptase (hTRT), the catalytic protein subunit of human telomerase. The polynucleotides and polypeptides of the invention are useful for diagnosis, prognosis and treatment of human diseases, for changing the proliferative capacity of cells and organisms, and for identification and screening of compounds and treatments useful for treatment of diseases such as cancers.
Owner:UNIV OF COLORADO THE REGENTS OF
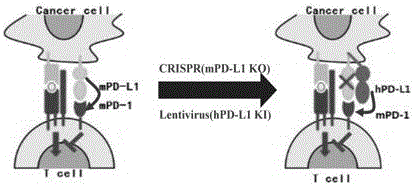
Humanized PD-L1 tumor cell line, animal model with same and application of humanized PD-L1 tumor cell line and animal model
ActiveCN105950560ASpeed up the processLethalCompounds screening/testingCell receptors/surface-antigens/surface-determinantsPD-L1Wilms' tumor
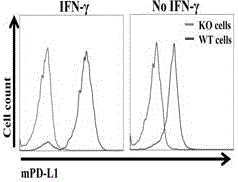
The invention provides a humanized PD-L1 tumor cell line MC-38-hPD-L1, a builtanimal tumor model with the same and a method for constructing the humanized PD-L1 tumor cell line. The method particularly includes knocking out animal-origin PD-L1 by the aid of CRISPR-CAS9; carrying out amplification and cultivation to obtain knocked-out cell banks; extracting DNA (deoxyribonucleic acid) and carrying out PCR (polymerase chain reaction) amplification; recycling and cloning amplification products; carrying out over-expression on human-origin PD-L1 in MC-38 cell lines of mPD-L1 KO by the aid of lentivirus systems; packaging lentivirus and screening Puromycin to obtain the humanized MC-38 cell line of PD-L1. The humanized PD-L1 tumor cell line, the animal tumor model and the method have the advantages that as shown by results, high killing efficiency and multiplication capacity are obviously presented by tumor infiltration CD8 T lymphocytes after antibody treatment is carried out, tumor infiltration Treg cells can be obviously inhibited after antibody treatment is carried out, and accordingly the method is proved to be effective and feasible from the aspect of molecular mechanisms.
Owner:SUZHOU INST OF SYST MEDICINE
Human telomerase catalytic subunit
InactiveUS20060040307A1Improve proliferative abilityOrganic active ingredientsFungiTelomerase Catalytic SubunitBiological body
The invention provides compositions and methods related to human telomerase reverse transcriptase (hTRT), the catalytic protein subunit of human telomerase. The polynucleotides and polypeptides of the invention are useful for diagnosis, prognosis and treatment of human diseases, for changing the proliferative capacity of cells and organisms, and for identification and screening of compounds and treatments useful for treatment of diseases such as cancers.
Owner:UNIV OF COLORADO THE REGENTS OF
Mammalian cells that have increased proliferative capacity
InactiveUS7195911B2Improve proliferative abilityHydrolasesPeptide/protein ingredientsTelomerase rnaTelomerase Reverse Transcriptase Protein
The present invention is directed to cells comprising a recombinant polynucleotide sequence that encodes a telomerase reverse transcriptase protein, variant, or fragment having telomerase catalytic activity when complexed with a telomerase RNA.
Owner:UNIV OF COLORADO THE REGENTS OF
HEK (human embryonic kidney) 293 cell line applicable to serum-free culture and application thereof
InactiveCN102604889AMicroorganism based processesViruses/bacteriophagesHEK 293 cellsVirulent characteristics
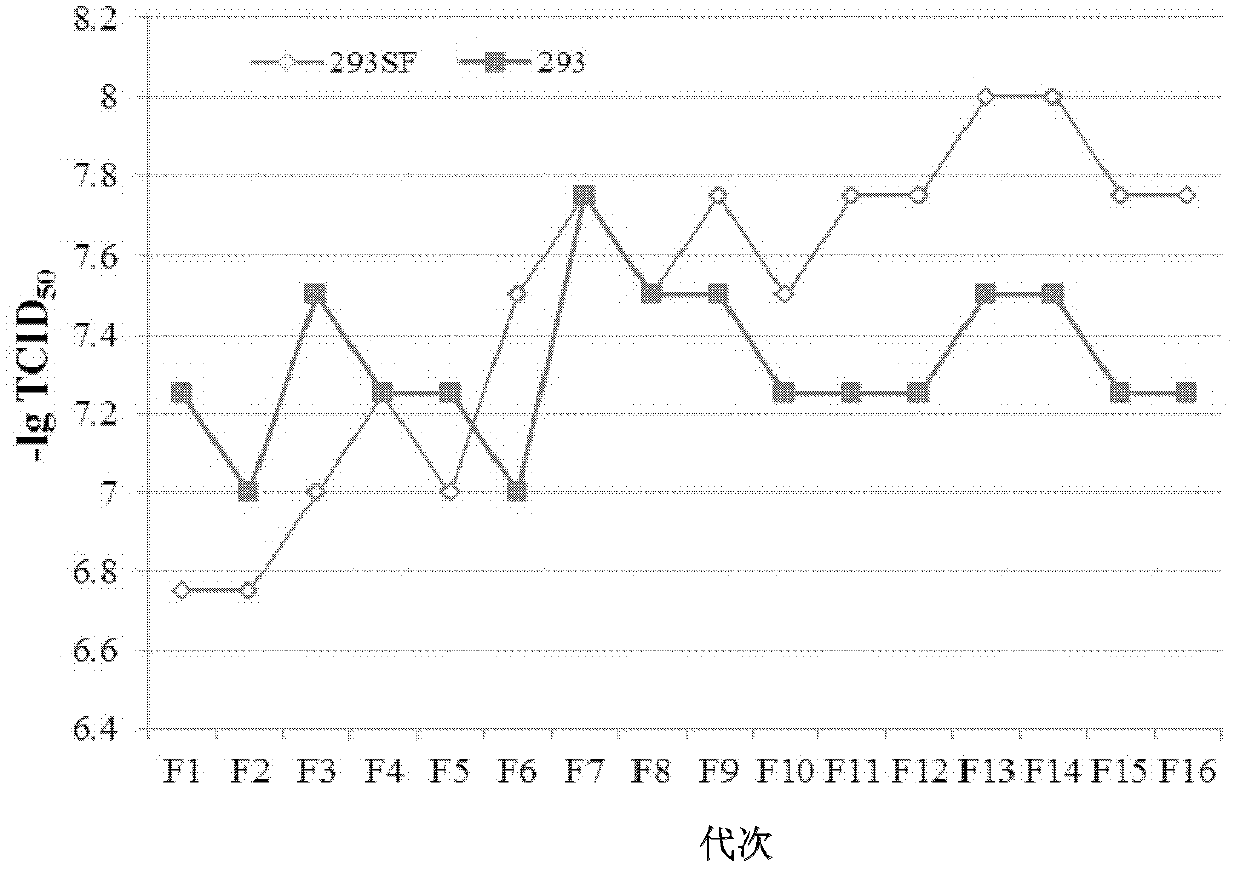
The invention discloses an HEK (human embryonic kidney) 293 cell line applicable to serum-free culture and an application thereof. According to the invention, the HEK 293 cell (293SF) applicable to serum-free culture is obtained by adopting a culture solution progressive substitution method, wherein the preservation number of the HEK 293 cell is CGMCC (China General Microbiological Culture Collection Center) No.5824. Detection shows that the 293SF cell has stable adenovirus proliferation capacity and foreign protein expressing ability; average lesion time is 97 hours and is reduced by 12.9% compared with the HEK 293 cell; virus virulence reaches up to 107.48TCID50 / mL and is improved by 43.3% compared with the HEK293 cell; and stability is good while generation number is improved, and the state and susceptibility of the 293SF cell are not changed after the 293SF cell is continuous passage is carried out for sixteen times. The cell line disclosed by the invention can be used for serum-free production of an adenovirus vector vaccine.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Isolation and use of solid tumor stem cells
InactiveUS20110092378A1Promote resultsPromotes significant proliferationDiagnosticsMicrobiological testing/measurementPrimary tumorImmunodeficient Mouse
A small percentage of cells within an established solid tumor have the properties of stem cells. These solid tumor stem cells give rise to both more tumor stem cells and to the majority of cells in the tumor that have lost the capacity for extensive proliferation and the ability to give rise to new tumors. Thus, solid tumor heterogeneity reflects the presence of tumor cell progeny arising from a solid tumor stem cell.We have developed a xenograft model in which we have been able to establish tumors from primary tumors via injection of tumors in the mammary gland of severely immunodeficient mice. These xenograft assay have allowed us to do biological and molecular assays to characterize clonogenic solid tumor stem cells.We have also developed evidence that strongly implicates the Notch pathway, especially Notch 4, as playing a central pathway in carcinogenesis.
Owner:RGT UNIV OF MICHIGAN
Improved mesenchyme stem cell protection solution and application thereof
ActiveCN101919380ACharacteristics unchangedMeet application needsDead animal preservationMesenchymeCell activity
The invention relates to an improved mesenchyme stem cell protection solution as well as application and a preparation method thereof. The protection solution can effectively prolong the activity remaining time of mesenchyme stem cells, reduces preparation cost, and has the advantages of wide raw material source, simple preparation, safe and reliable direct clinical application; and after the mesenchyme stem cells are preserved for 48 hours in the protection solution, the cell activity is still above 90 percent, the cell morphology is normal, and the multiplication capacity and mesenchyme stem cell phenotype characteristics are not influenced.
Owner:青岛奥克生物开发有限公司
Isolation and expansion of human marrow stromal cells
The invention includes in vitro methods of inducing and enhancing proliferation of human marrow stromal cells for use in, for example, gene therapy and transplantation methods. The invention also includes a method of assessing the expandability (i.e., proliferative capacity) of human marrow stromal cells. In addition, the invention includes a conditioned medium for enhancing proliferation of human marrow stromal cells.
Owner:PHILADELPHIA HEALTH & EDUCATION CORP
Cultural method for inducing human embryonic stem cell to directionally differentiate into corneal limbal stem cell
ActiveCN102952779AGood in vitro differentiation and proliferation abilitySuppress generationArtificially induced pluripotent cellsNon-embryonic pluripotent stem cellsImmunofluorescenceMicroscopic observation
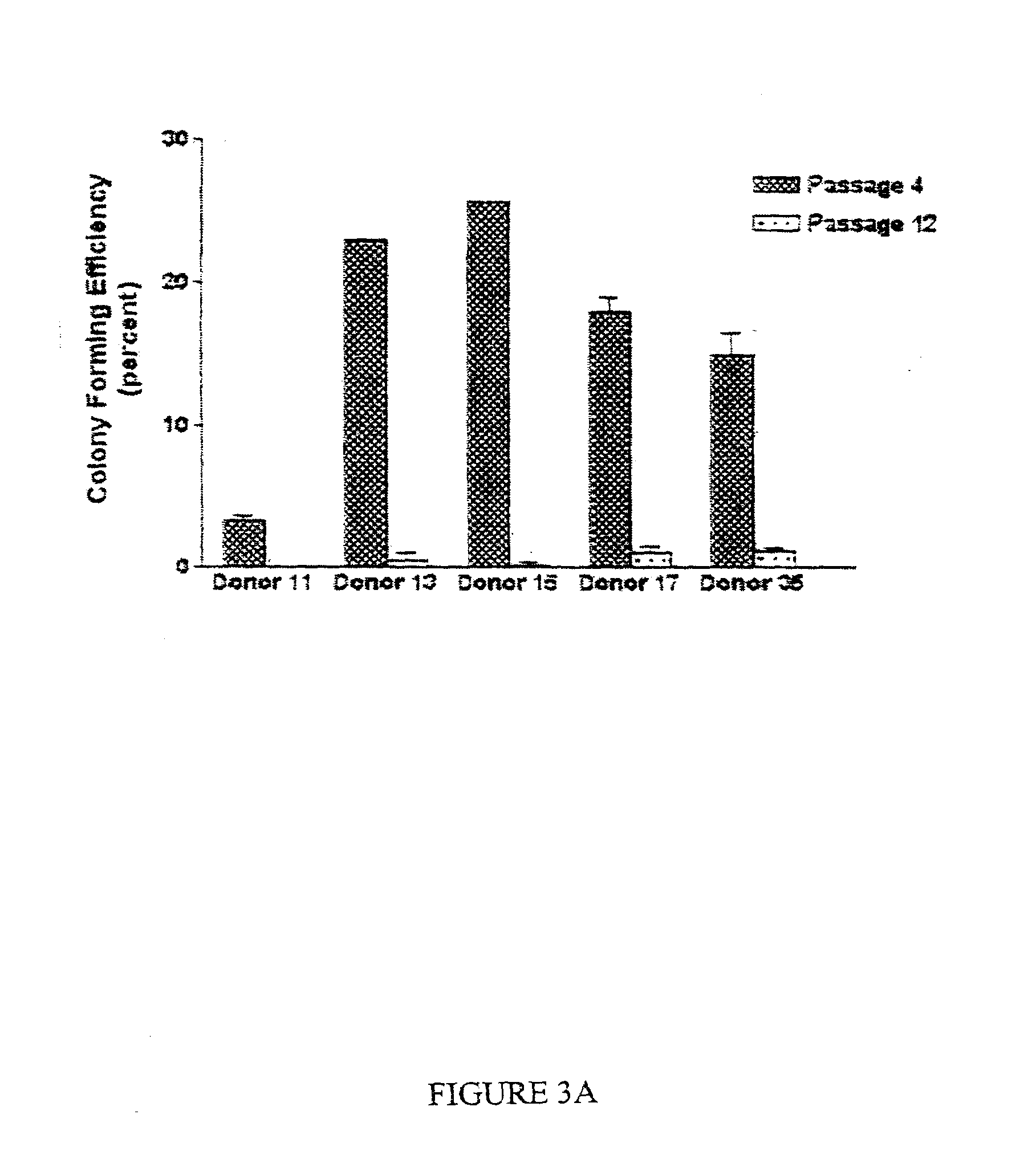
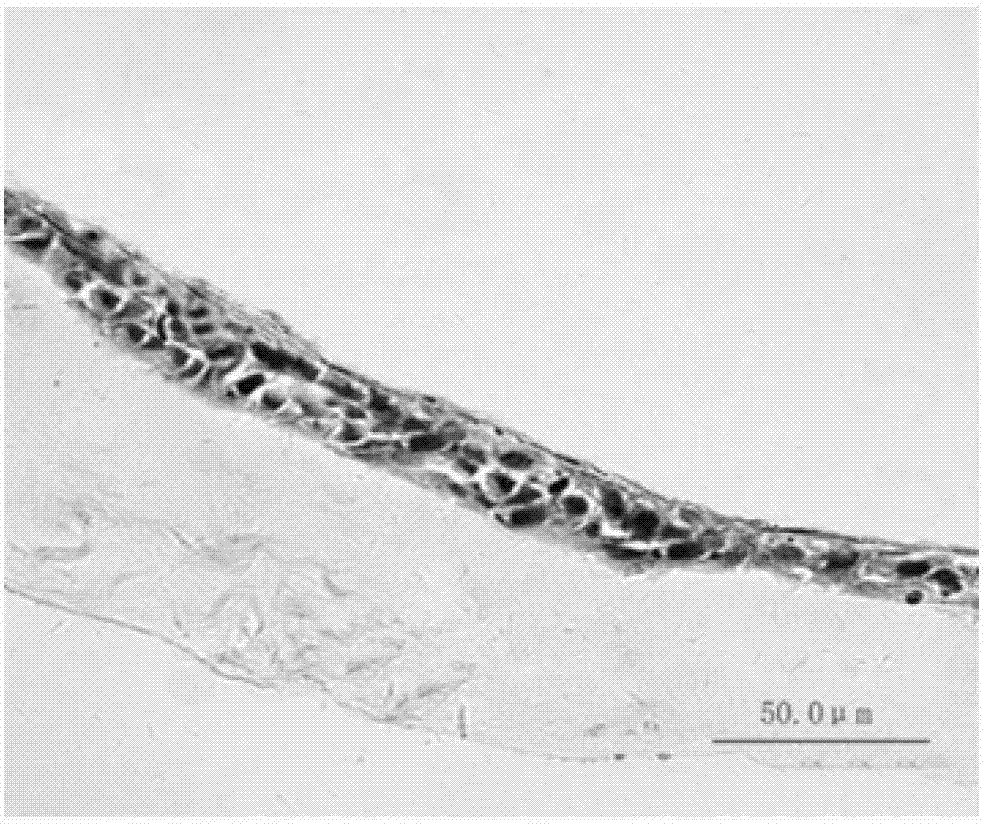
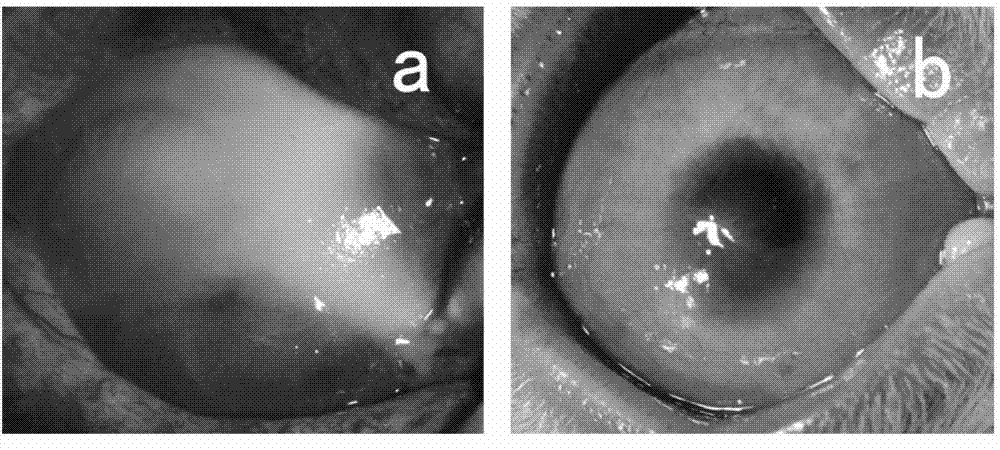
The invention discloses a cultural method for inducing a human embryonic stem cell to directionally differentiate into a corneal limbal stem cell, which comprises the following steps that firstly, a DMEM (Dulbecco's Modified Eagle Medium) / F12 conditioned medium is adopted to culture a human primary corneal limbal stem cell to prepare a corneal limbal stem cell conditioned medium, and then the corneal limbal stem cell conditioned medium is utilized and combined with IV type collagen culture in vitro to induce the human embryonic stem cell to directionally differentiate into the corneal limbal stem cell. According to the corneal limbal stem cell obtained by utilizing the cultural method, through light microscope observation in vitro, electron microscope observation, real-time quantitative polymerase chain reaction, immunofluorescence, flow cytometry, cloning efficiency determination and the like, the induced cell has a similar shape and phenotype with a normal corneal limbal stem cell, has good differentiation and proliferation capacity in vitro, can be transferred in vitro for more than four generations and can be used as a seed cell for preparing a corneal graft.
Owner:SHANDONG UNIV
Serum-free medium for placenta-derived mesenchymal stem cells and preparation method thereof
ActiveCN105112362AImprove securityImprove proliferative abilitySkeletal/connective tissue cellsINSULIN HUMANProliferative capacity
The invention belongs to the technical field of stem cells and relates to a serum-free medium for placenta-derived mesenchymal stem cells and a preparation method thereof. The serum-free medium comprises a DMEM basic medium and further comprises vitamin H, glutathione, recombinant human insulin, human serum albumin, transferrin, fibroblast growth factors, epidermal growth factors, stem cell growth factors, Human stem cell factors and magnolol. The serum-free medium provided by the invention has the advantages of high safety and capability for significant improvement of proliferative capability of placenta-derived mesenchymal stem cells, the serum-free medium is capable of well keeping cell forms and stem cell characteristics of the placenta-derived mesenchymal stem cells and is convenient to popularize and apply.
Owner:广东美赛尔细胞生物科技有限公司
Fourth-generation CAR-T cell as well as construction method and application thereof
ActiveCN109504660AReduce cancer recurrencePromote proliferationAntibody mimetics/scaffoldsMammal material medical ingredientsSurvivabilityLymphatic Spread
The invention provides construction and application of a fourth-generation chimeric antigen receptor T (CAR-T) cell (expressing IL-7 and CCL19) specific to Nectin-4 dual targets on the surface of a malignant tumor. Two spatial epitopes of a Nectin-4 antigen are taken as target points, and the constructed fourth-generation CAR-T is used for treating and expressing a malignant solid tumor of the Nectin-4 antigen to solve the immune escape problem of the CAR-T during treatment of the solid tumor; and the constructed fourth-generation CAR-T can enhance proliferative capacity and sustained survivability, so that the solid tumor is effectively treated, and a new strategy is provided for effective prevention and treatment of postoperative recurrence / metastasis of the solid tumor.
Owner:温州启星生物技术有限公司
Support for tissue regeneration and process for producing the same
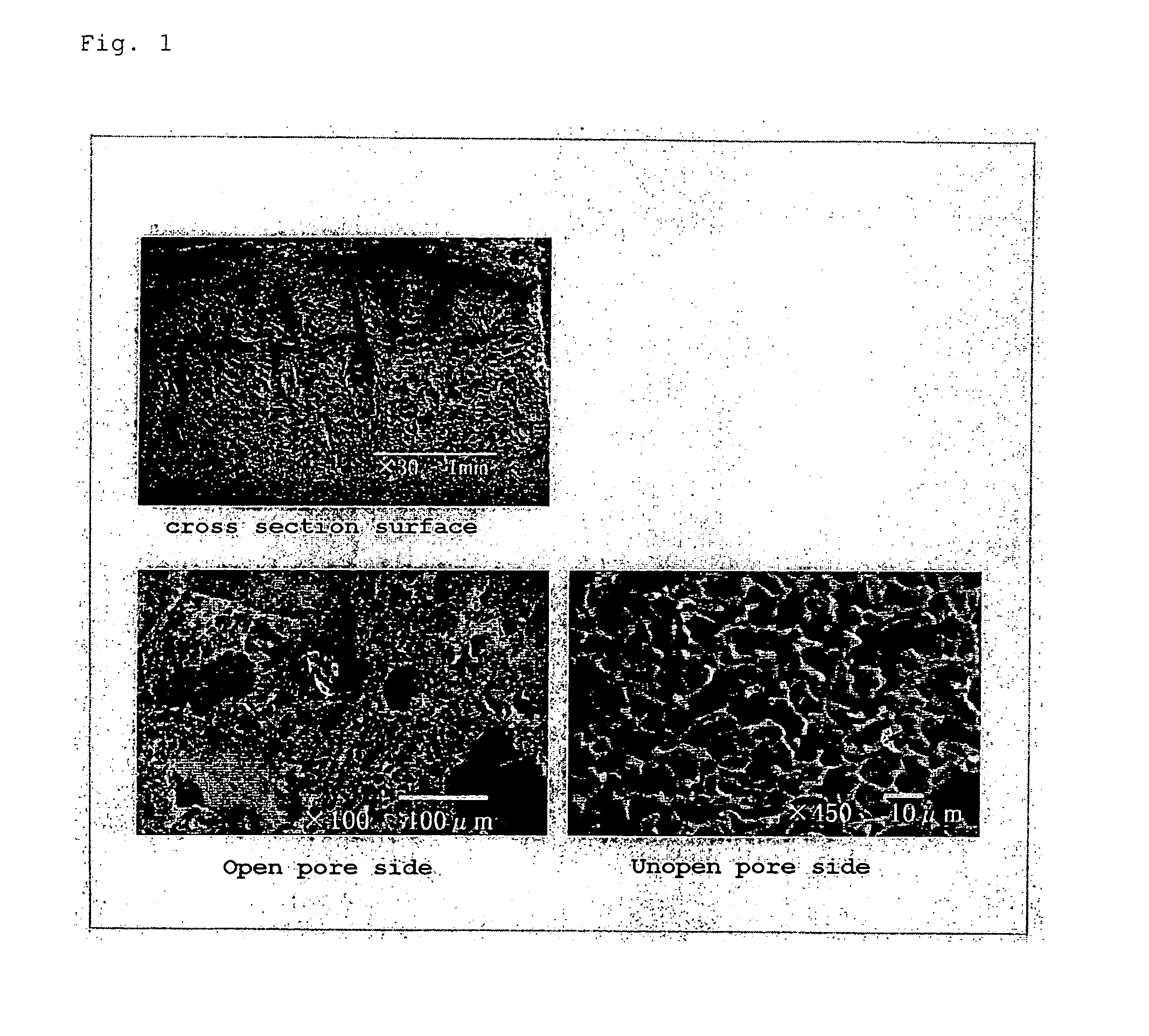
ActiveUS20060127368A1Improve retentionExcellent in grafting abilityBiocideMammal material medical ingredientsFreeze-dryingMedical treatment
The present invention provides a scaffold in which cells can be stably retained and grafted in a uniform distribution state in the culture, preferable proliferation ability and viability can be secured, and particularly in the case of cartilage, fixation treatment such as suture can be carried out in the transplantation into affected parts after the culture, and the mechanical strength is provided sustainable for (weighted) compression at the initial stage of transplantation. The present invention relates to a 3-dimensional porous scaffold for tissue regeneration which comprises a structure composed of vertically long-shaped pores having a pore diameter of not less than 10 μm to not more than 500 μm and pore length of not less than 20 μm to not more than 1 cm being juxtaposedly arranged obtained by a production process comprising rapid freeze-drying as a key technology. Further, the invention relates to the above 3-dimensional porous scaffold in which seeding properties of cells are improved by a pore enlargement treatment of one side face by a separation operation, a salt elution operation of a surface part, or a combination of these operations. It becomes possible to produce a 3-dimensional cell combination having excellent degree of tissue formation and medical treatment effect by seeding a cell or precursor cell derived from a tissue in this scaffold, and culturing them in an artificial environment and / or the living body.
Owner:GC CORP +2
Kit for detection of telomerase reverse transcriptase nucleic acids
InactiveUS20090269739A1Improve proliferative abilityOrganic active ingredientsSenses disorderBiological bodyReverse transcriptase
The invention provides compositions and methods related to human telomerase reverse transcriptase (hTRT), the catalytic protein subunit of human telomerase. The polynucleotides and polypeptides of the invention are useful for diagnosis, prognosis and treatment of human diseases, for changing the proliferative capacity of cells and organisms, and for identification and screening of compounds and treatments useful for treatment of diseases such as cancers.
Owner:GERON CORPORATION +1
Preparation method of CIK (Cytokine Induced Killer) cells with high proliferation capacity, high cytotoxic activity and high survival rate, associated CIK cells and application
InactiveCN102827809AImprove proliferative abilityHighly toxic activityMammal material medical ingredientsBlood/immune system cellsPeripheral blood mononuclear cellClinical efficacy
The invention relates to a preparation method of CIK (Cytokine Induced Killer) cells with high proliferation capacity, high cytotoxic activity and high survival rate. The preparation method comprises the steps of: (1) sorting and removing CD4<+>CD25<+>Treg cells of peripheral blood mononuclear cell to obtain CIK pre-cells; (2) cultivating the CIK pre-cells in a cell culture fluid containing 100ng / ml of PHA, 100ng / ml of IL-6 and 10ng / ml of PGE2 for 24h; (3) transferring the CIK pre-cells to a cell culture bottle coated with 1microgramme / ml of CD3 monoclonal antibody, and adding 1000U / ml of IFN-gamma for cultivating for 48h; (4) adding 1000U / ml of IL-2 and 100ng / ml of IL-1alpha for cultivating for 4 days; and (5) adding 1microgramme / ml of insulin to continuously cultivate for 7-14 days. The invention further provides associated CIK cells, a method for inhibiting the peripheral blood mononuclear cell to be differentiated to the CD4<+>CD25<+>Treg cells and a method for promoting the proliferation of the CIK cells. The preparation method of the CIK cells provided by the invention is skillful in design, and the tumor killing cells-CIK cells prepared have stronger proliferation capacity, higher cytotoxic activity and better tumor killing efficiency, so that the clinical efficacy is improved, and the preparation method is appropriate for wide application in a large scale.
Owner:上海优立赛尔生物医药科技有限公司
Separation and culturing method of human epidermis stem cell
Owner:陕西艾尔肤组织工程有限公司
Methods for extending the replicative capacity of somatic cells during an ex vivo cultivation process
InactiveUS20190024079A1Improve proliferative abilityReplication capacity can be improvedHydrolasesGenetically modified cellsTelomeraseADAMTS Proteins
Owner:UPSIDE FOODS INC
Methods for detection of human telomerase reverse transcriptive protein
InactiveUS7413864B2Improve proliferative abilityPeptide/protein ingredientsAntibody mimetics/scaffoldsBiological bodyReverse transcriptase
The invention provides compositions and methods related to human telomerase reverse transcriptase (hTRT), the catalytic protein subunit of human telomerase. The polynucleotides and polypeptides of the invention are useful for diagnosis, prognosis and treatment of human diseases, for changing the proliferative capacity of cells and organisms, and for identification and screening of compounds and treatments useful for treatment of diseases such as cancers.
Owner:UNIV OF COLORADO THE REGENTS OF
Muteins of human telomerase reverse transcriptase lacking telomerase catalytic activity
InactiveUS7517971B1Improve proliferative abilityOrganic active ingredientsSenses disorderMutated proteinNucleotide
The invention provides compositions and methods related to human telomerase reverse transcriptase (hTRT), the catalytic protein subunit of human telomerase. The polynucleotides and polypeptides of the invention are useful for diagnosis, prognosis and treatment of human diseases, for changing the proliferative capacity of cells and organisms, and for identification and screening of compounds and treatments useful for treatment of diseases such as cancers.
Owner:UNIV OF COLORADO THE REGENTS OF
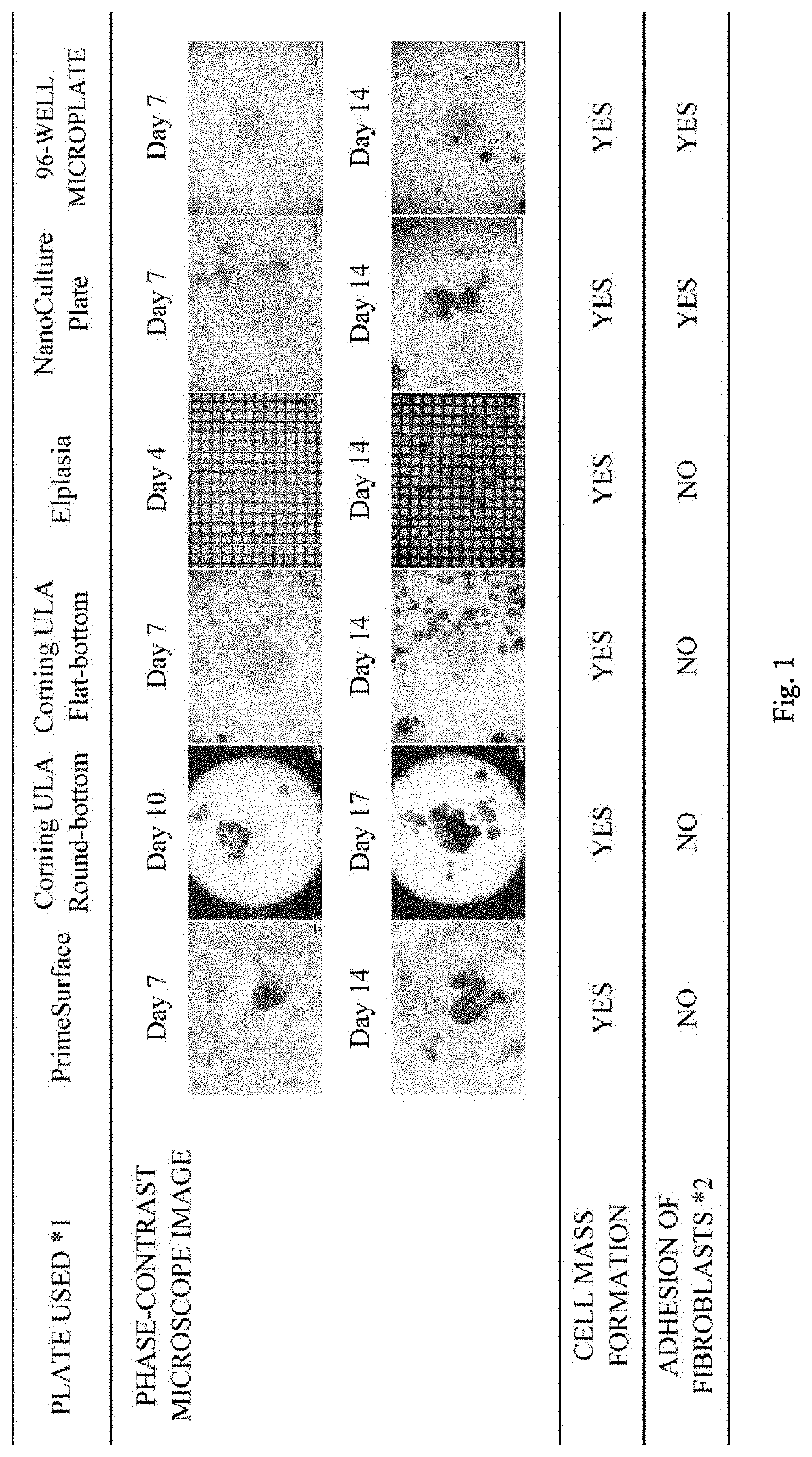
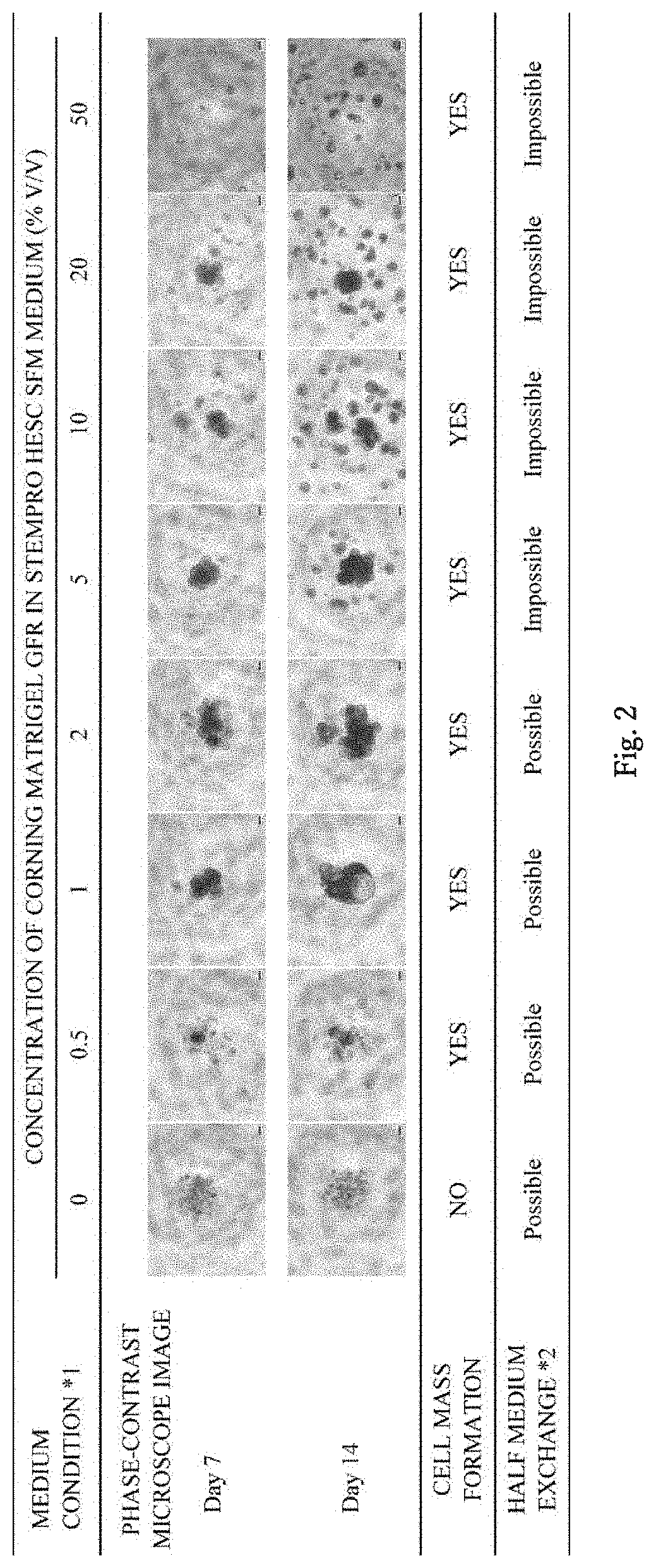
Three-dimensional culture of primary cancer cells using tumor tissue
ActiveUS20200071676A1Improve throughputPrevent proliferationCell culture supports/coatingBiological testingCancer cellCell-Extracellular Matrix
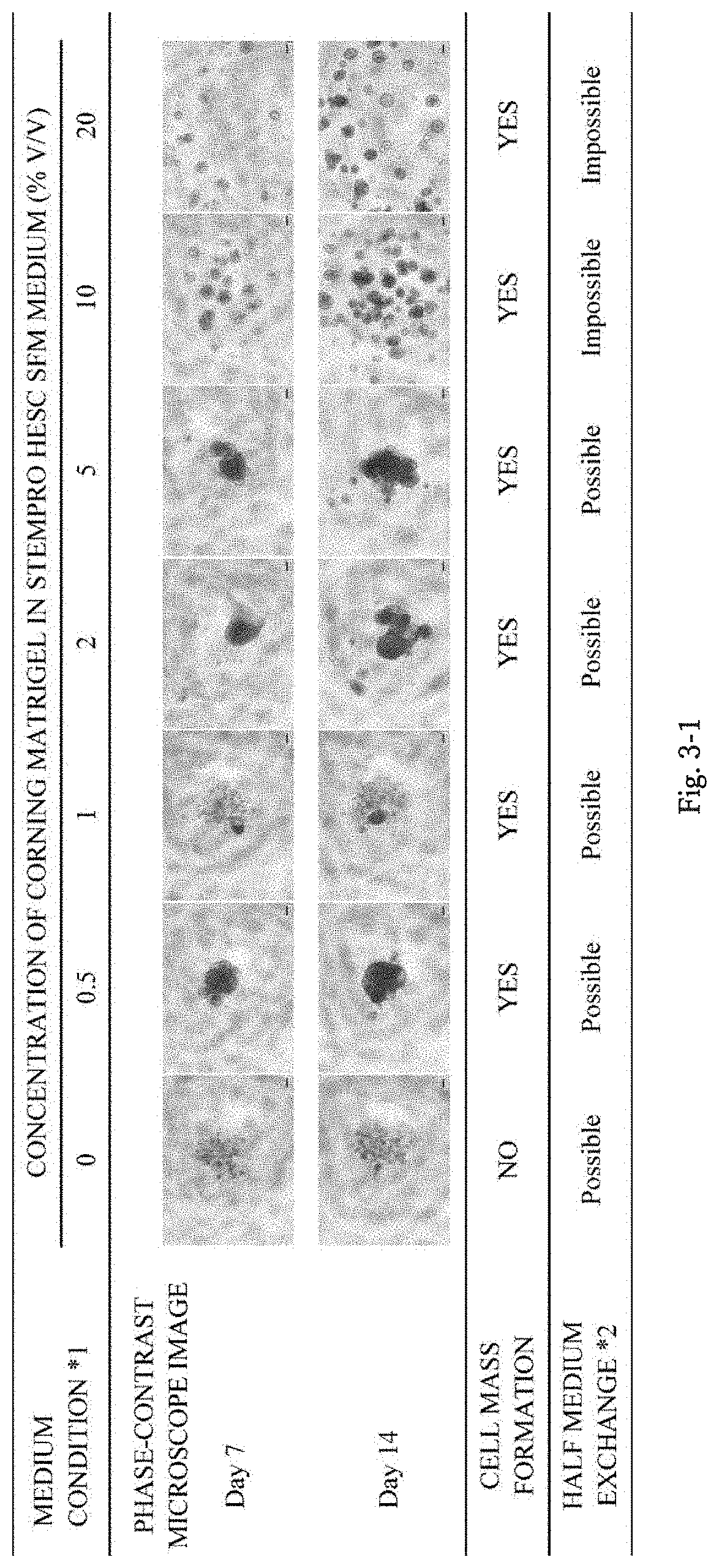
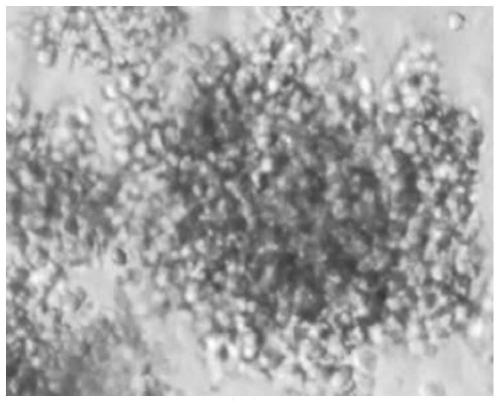
A method of producing a cell mass by three-dimensional culture of primary cancer cells having proliferative ability and properties of handleability, versatility, and high-throughput performance, in which a tumor tissue is used as a starting material, proliferation of cells such as fibroblasts other than cancer cells is inhibited, and the cell mass includes primary cancer cells as a main component. The object is achieved by providing a method of producing a cell mass by three-dimensional culture of primary cancer cells using a tumor tissue, including: a three-dimensional culture step of culturing cells obtained from the tumor tissue in a medium containing a 5% v / v or less extracellular matrix on a substantially low-adhesive cell culture substrate.
Owner:MITSUBISHI CHEM MEDIENCE
Enhanced NK cell with strong immune regulation and strong killing capacity on tumor cells and virus infected cells, preparation method and kit thereof
InactiveCN111394310ADomesticated immunomodulatory capacityImprove proliferative abilityCulture processSkeletal/connective tissue cellsCD16Natural Killer Cell Inhibitory Receptors
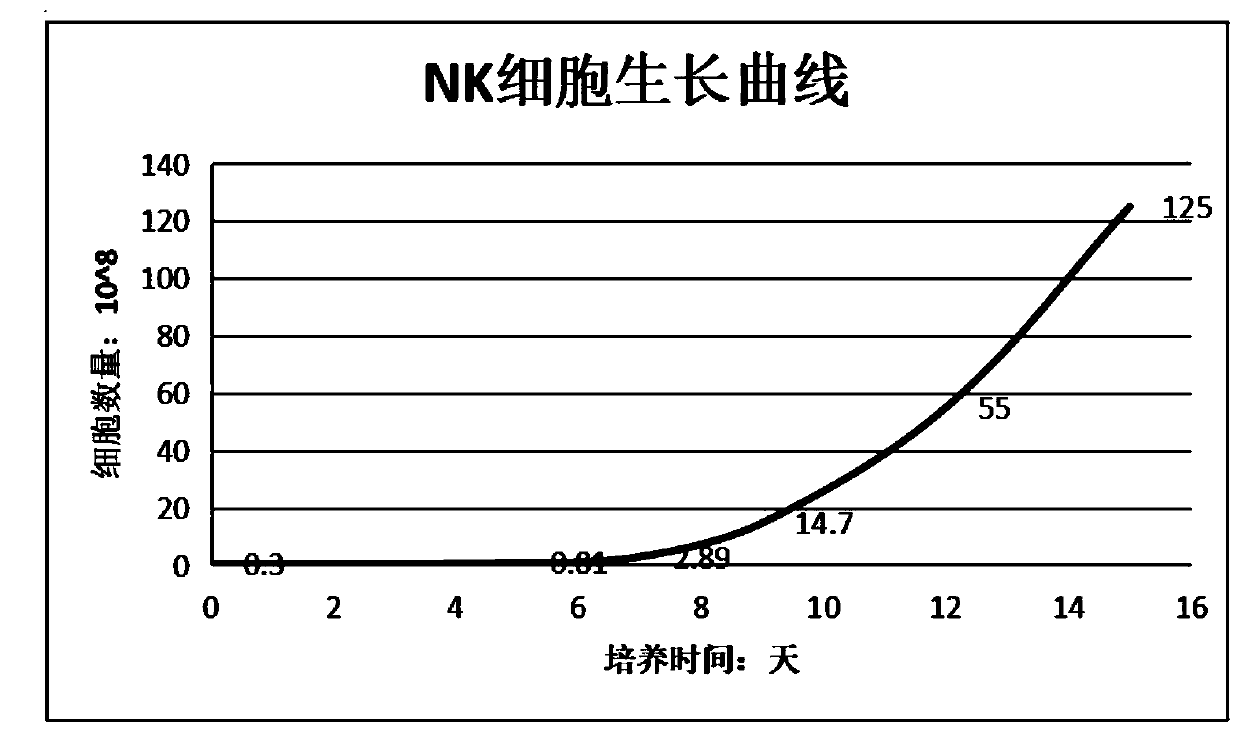
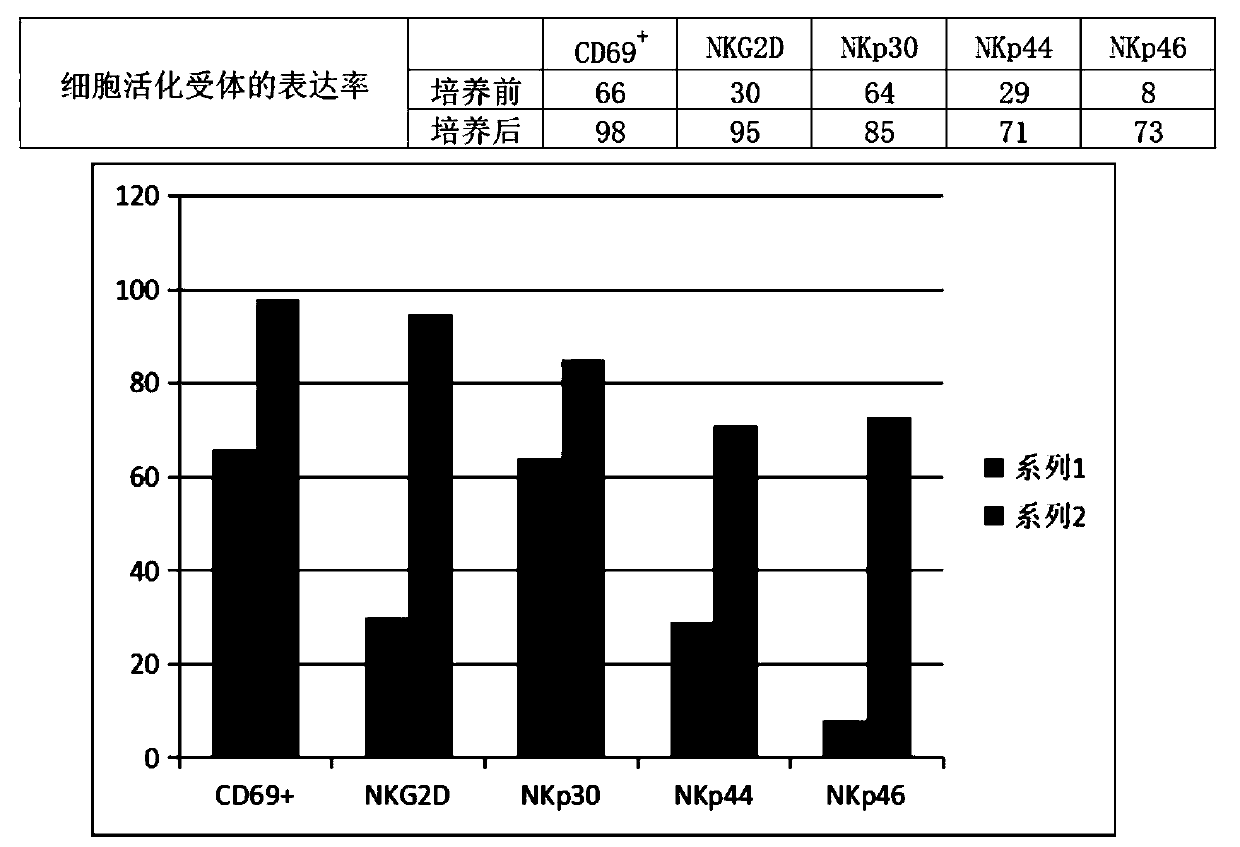
The invention discloses an enhanced NK cell with strong immune regulation and strong killing capacity on tumor cells and virus infected cells, a preparation method and a preparation kit thereof. A Transwell culture system is adopted to use mesenchymal stem cells to incubate and acclimate NK cells before activation, so that the Transwell culture system co-cultures the mesenchymal stem cells and theNK cells, and the immune regulation ability of the NK cells is acclimated. In addition, the NK cells are activated and cultured by CD16 monoclonal antibodies combined with IFN-gamma, OK432, IL-2, IL-21 and IL-15, and the NK cells are amplified and cultured by IL-2 and nicotinamide, so that the proliferation ability of the NK cells is enhanced, and the killing activity of the NK cells on the tumorcells and the virus infected cells is promoted.
Owner:北京荟科柘生物科技有限公司
Efficient and fast method for separating mesenchymal stem cells
InactiveCN102851255AHigh activityProliferation effect is goodSkeletal/connective tissue cellsEnzyme digestionBiological property
The invention discloses a fast and efficient biological technical method for separating and amplifying umbilical cord mesenchymal stem cells. According to the method, umbilical cord tissues from full-term fetus born by cesarean section are utilized to obtain a large amount of mesenchymal stem cells through a mixed enzyme digestion method combined with an explant method, in a short period of time. This method effectively shortens enzyme digestion time, reduces loss of cell number to a largest degree, and improves the influence on biological activity of mesenchymal stem cells in a long enzyme digestion process. The method also optimizes in vitro amplification conditions; and the amplified cells have the biological characteristics of mesenchymal stem cells and high proliferation ability. The invention creates a fast and efficient biological technology for separation, purification and amplification of human umbilical cord mesenchymal stem cells, and provides an adequate seed cell source for research and clinical application of stem cells.
Owner:张兆光 +2
Cell freezing medium
The invention discloses a cell freezing medium. The cell freezing medium comprises a basal culture medium, glycerin, fetal calf serum and a non-permeable protective agent, and the contents of the components are 80-94 v / v% of the basal culture medium, 5-10 v / v% of glycerol, 1-10 v / v% of the fetal calf serum and 0.01-0.10 w / v% of the non-permeable protective agent, and the basal culture medium comprises RPMI-1640, MEM, high-sugar DMEM, low-sugar DMEM or DMEM-F12; and the non-permeable protective agent comprises sodium alginate, sodium hyaluronate or gamma-polyglutamic acid. Dimethyl sulfoxide isnot contained, a good freezing effect is achieved through different matching of the non-permeable protective agent with glycerin and fetal calf serum, a strong water molecule complexing ability is achieved, the freezing function is similar to the freezing function of dimethyl sulfoxide, but formation of intracellular ice crystals is reduced during freezing; the cell freezing medium has clear added ingredients, high safety, small damage to cells, consistent morphology before and after freezing, a high cell survival rate after cell resuscitation and good proliferative capacity.
Owner:EAST CHINA UNIV OF SCI & TECH
Phospholipid scramblase 3
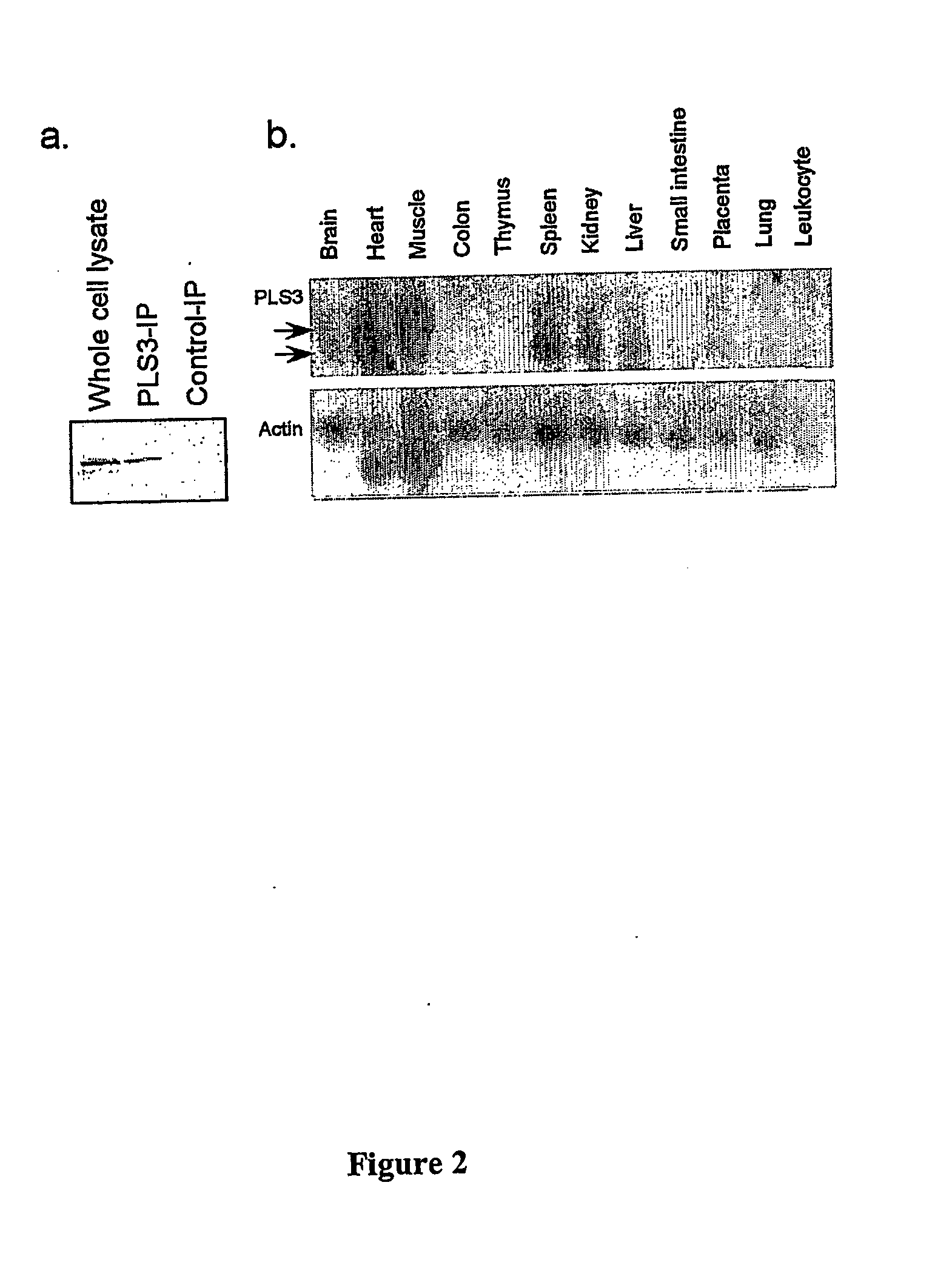
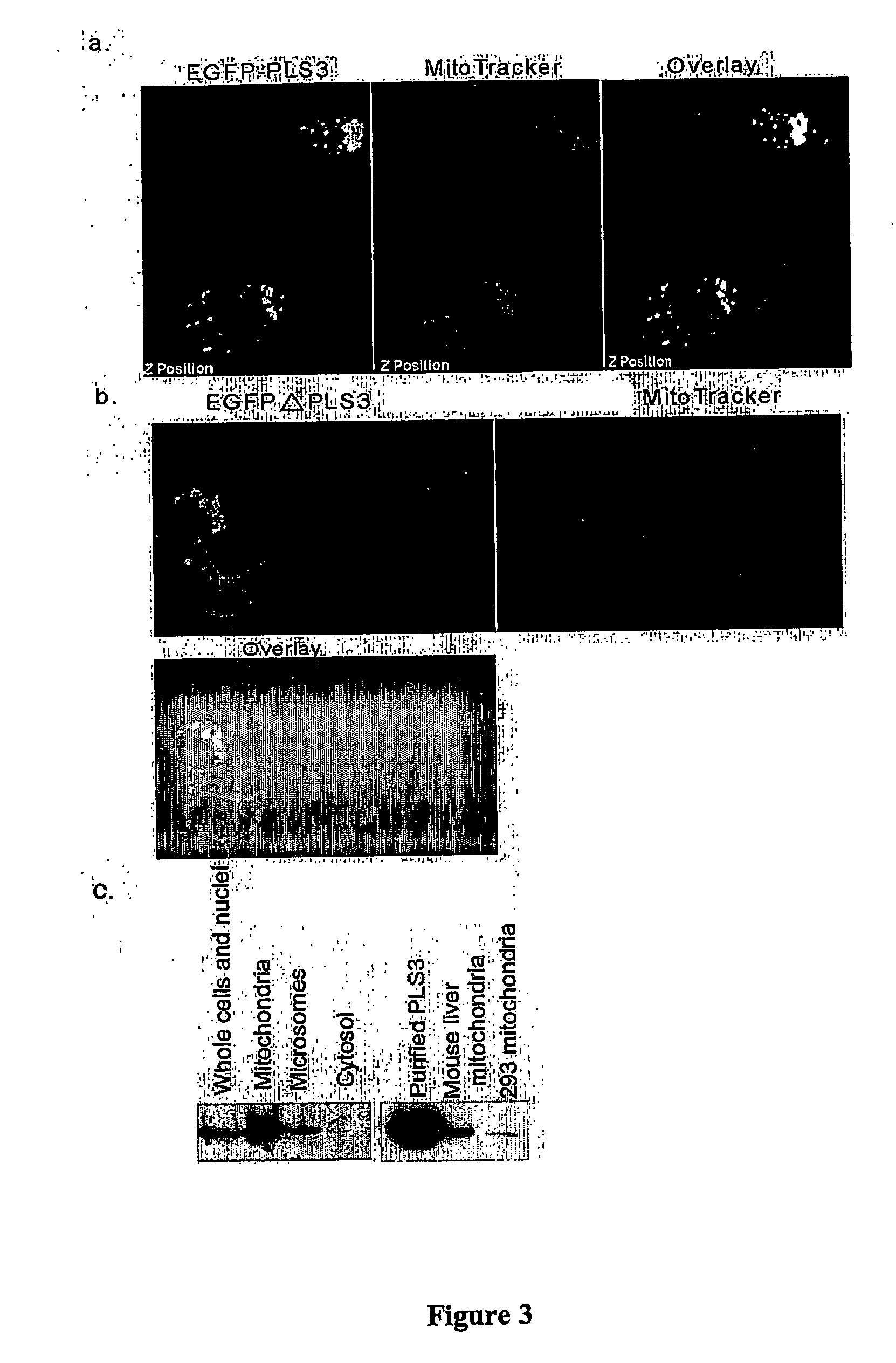
InactiveUS20060172958A1DisruptedInduce cell deathSugar derivativesGenetic material ingredientsLipid formationProliferative capacity
Phospholipid scramblase 3 (PLS3) is a newly recognized member of a family of proteins responsible for phospholipid translocation between two lipid compartments. A novel isoform of PLS3 is identified and characterized herein. The function of PLS3 in mitochondria was disrupted, yielding an inactive mutant PLS3(F258V). Cells transfected with PLS3(F258V) exhibited reduced proliferative capacity that was unaffected by the presence of Na3N. PLS3(F258V)-expressing cells exhibit abnormal mitochondrial metabolism and structure and were associated with decreased sensitivity to UV- and tBid-induced apoptosis, and diminished translocation of cardiolipin to the outer mitochondrial membrane. Cells transfected with wild-type PLS3 displayed increased sensitivity to apoptosis and enhanced cardiolipin translocation. These studies identify PLS3 as a regulator of mitochondrial structure and respiration, and cardiolipin transport in apoptosis.
Owner:UNIV OF UTAH RES FOUND
Promoter regions of the mouse and human telomerase RNA component genes
InactiveUS7084267B1Increase and decrease promoter activityIncrease and decrease magnitudeOrganic active ingredientsFungiTelomerasePromoter activity
The present invention relates to the identification of the genomic promoter region of the human and mouse telomerase RNA gene. Telomerase activity is necessary for the unrestricted proliferative capacity of many human cancers. It is proposed that mutation or dysregulation of the telomerase repression pathway may cause reactivation or upregulation of telomerase expression in cancer. The invention provides details of elements important for the regulation of telomerase RNA genes, including the Sp family of transcription factors. There is further provided methods for screening elements having the ability for suppressing telomerase RNA gene promoter activity and use of such elements in the treatment of cancers. In addition, evidence is also provided for the development of new transcription based therapies for cancer and for genetic approaches to targeting therapeutic genes to cancer cells. Namely, (1) transcriptional repression and the disruption of signal transduction pathways regulating telomerase activation. (2) Tumour specific gene expression for genetic therapy via telomerase RNA gene promoters.
Owner:THE UNIV COURT OF THE UNIV OF GLASGOW
Method for screening and culturing extracellular hair follicle stem cell matrix for clinic treatment level cell therapy
InactiveCN105255822AConvenient for clinical operationExtended transplant timeArtificially induced pluripotent cellsNon-embryonic pluripotent stem cellsCell-Extracellular MatrixECM Protein
The invention discloses culturing, screening and amplifying techniques for hair follicle stem cells for clinic treatment level cell therapy. The bottlenecks are always research hotspots and difficulties in the biological stem cell field; currently, by carrying out separation and purification by virtue of remarkable adhering properties of a basement membrane, the hair follicle stem cells are obtained by virtue of three-dimensional culture and amplification measures. By depending on amplification anchorage-dependent cells, a three-dimensional high-simulation in-vivo extracellular matrix system is provided for screening and separating the levels of target cells. The method is a novel culture technique for culture in vitro of biological cells of stem cells of adults (embryo skin) in a high-simulation in-vivo adhesion environment. Skin seed hair follicle cells of targeted tissue engineering cultured through adhesion screening in the environment of a high-simulation substrate have high abdication capacity and multiplication capacity and can form a differentiated cortex structure in vitro. By screening immunogenic cells, immune escape is safely achieved, the transplant time is prolonged, the clinic application of tissue engineering is increased, the healing of wounds is promoted, and a solution with absolute advantages for the clinic treatment level cell therapy is provided for diseases of skin.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Human telomerase reverse transcriptase polypeptides
InactiveUS7622549B2Improve proliferative abilityPeptide/protein ingredientsAntibody mimetics/scaffoldsNucleotideReverse transcriptase
The invention provides compositions and methods related to human telomerase reverse transcriptase (hTRT), the catalytic protein subunit of human telomerase. The polynucleotides and polypeptides of the invention are useful for diagnosis, prognosis and treatment of human diseases, for changing the proliferative capacity of cells and organisms, and for identification and screening of compounds and treatments useful for treatment of diseases such as cancers.
Owner:GERON CORPORATION +1
Lentiviral vector with efficient infectivity and multiplication capacity promoting effect for T cells and hematopoietic stem cells
ActiveCN104892770AHigh infection efficiencyPromote proliferationConnective tissue peptidesDepsipeptidesVirus typeT cell
The invention provides a method for constructing a lentiviral vector with efficient transfection capacity and efficient bioactivity expression capacity. Experimental results show that the viral infection efficiency can be increased by 30-70% by using the method; in addition, the inventor unexpectedly finds that the proliferation of the T cells can also be effectively stimulated by using the technical scheme, and furthermore the treatment process of CAR-T cells is simplified, and the cost is reduced.
Owner:SHANGHAI JI KAI GENE TECH CO LTD
Stem cell cryopreservation liquid and cryopreservation method
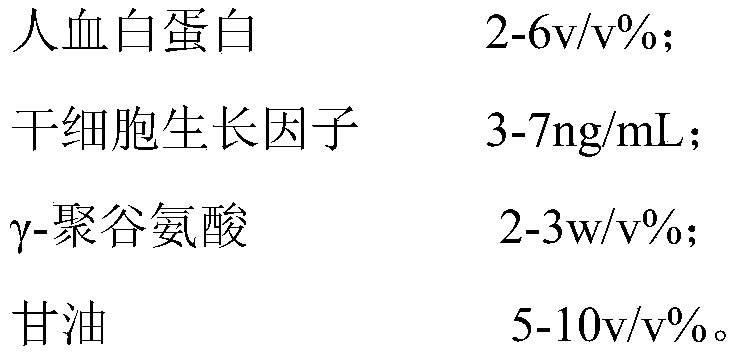
InactiveCN111418580AReduce formationImprove freezing effectDead animal preservationProliferative capacityPolyglutamic acid
The invention belongs to the field of cells and bioengineering, and particularly relates to a stem cell cryopreservation liquid and a cryopreservation method. The stem cell cryopreservation liquid iscomposed of a main component and an additive component, the main component is PBS, normal saline or a basal culture medium, and the additive component is human serum albumin, stem cell growth factors,gamma-polyglutamic acid and glycerin. A stem cell suspension is centrifuged, supernatant is removed, the product is mixed with the stem cell cryopreservation liquid, and subpackaging and cryopreserving are carried. According to the invention, DMSO and FBS are not contained; gamma-polyglutamic acid glycerol is matched differently; the cryopreservation liquid can obtain better freezing effect by combining with human serum albumin, has strong water molecule complexing capacity, can reduce the formation of ice crystals in cells in the freezing process, can be used for cryopreservation of cells, and has the positive effects of consistent forms before and after cell cryopreservation, high survival rate after cell resuscitation and good proliferation capacity of cells after cryopreservation resuscitation.
Owner:山东万能干细胞生物技术有限公司
Cell therapeutic agent for cancer treatment and combination therapy with same
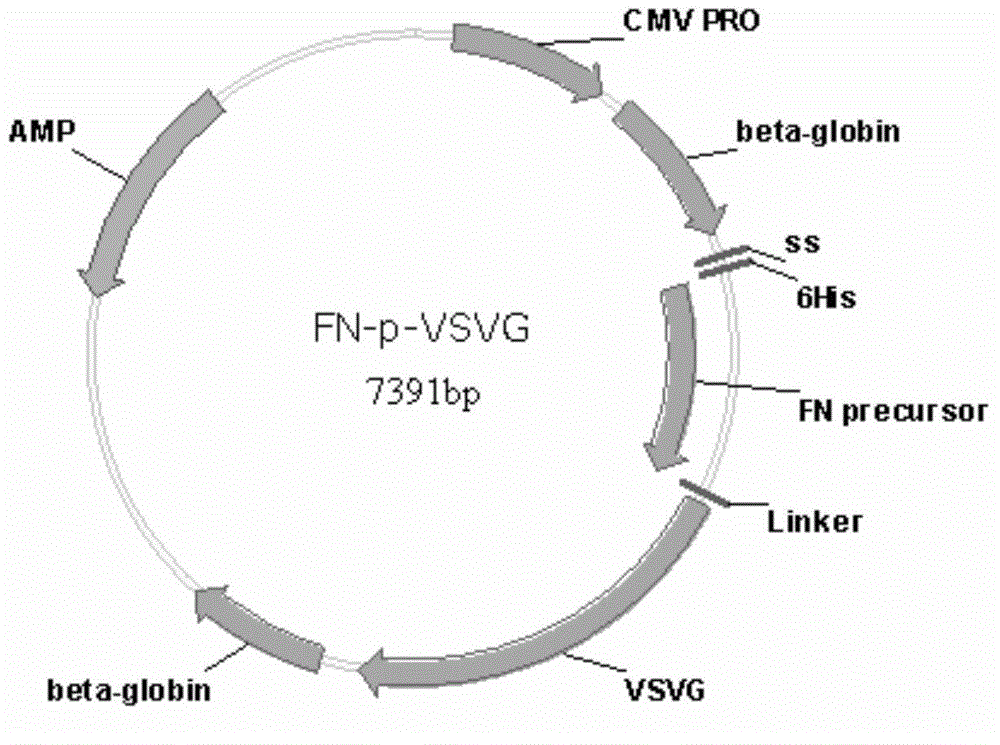
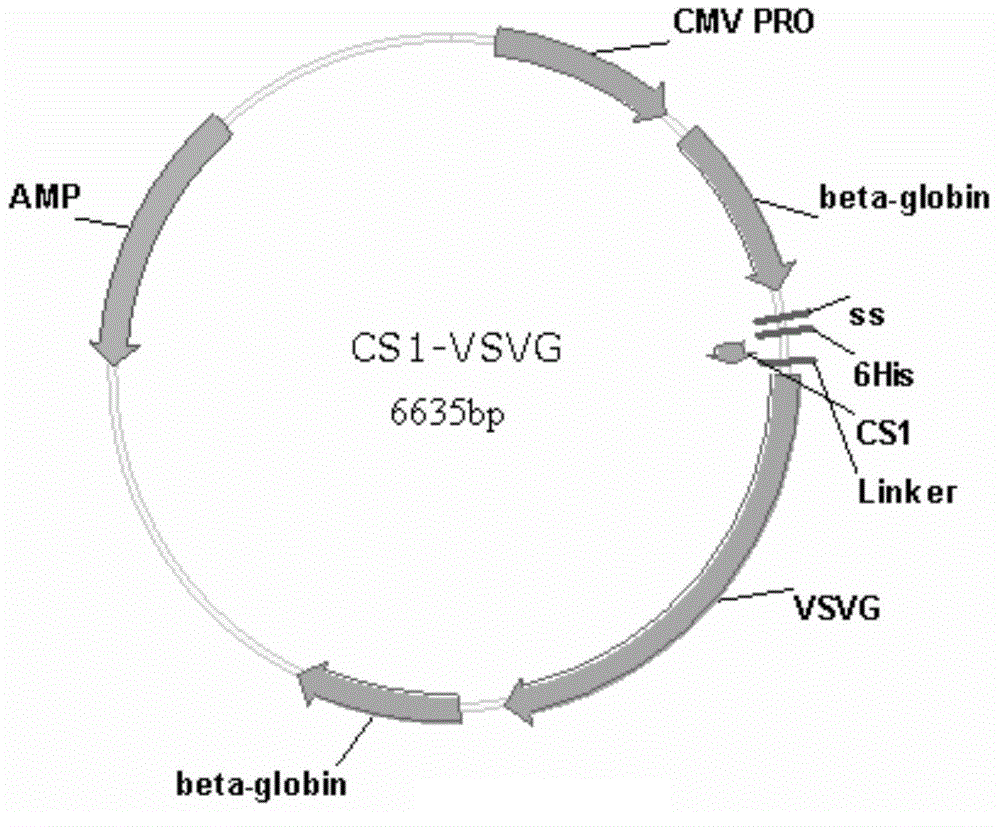
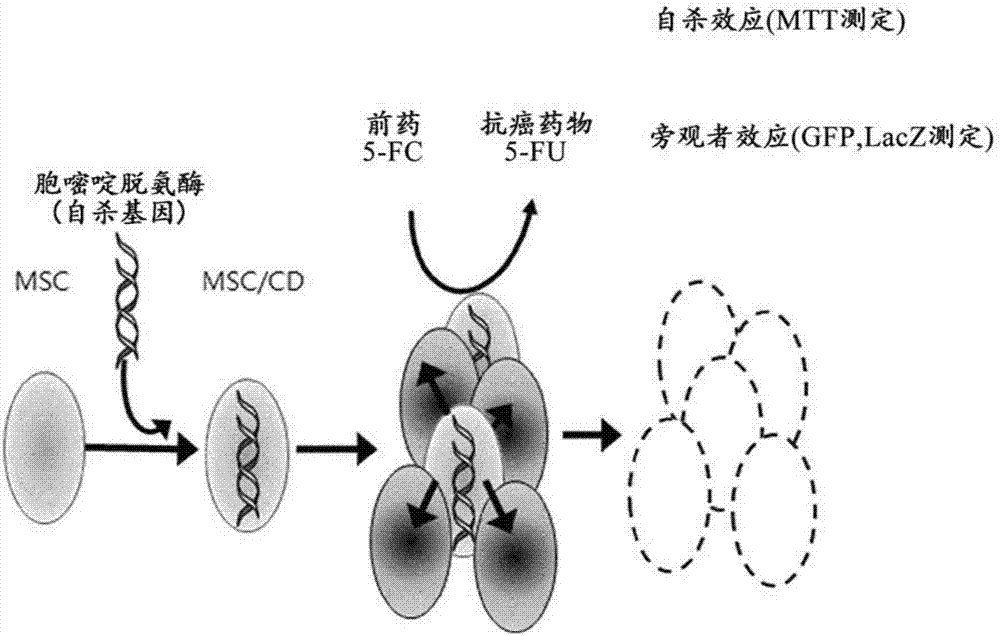
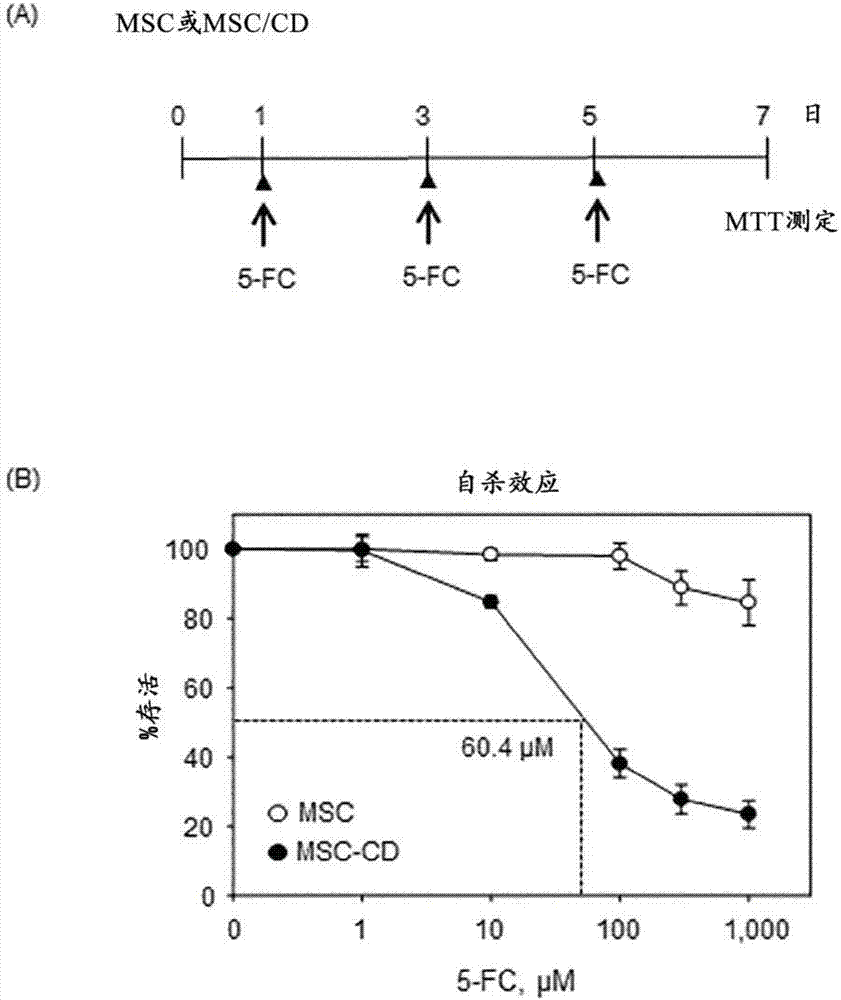
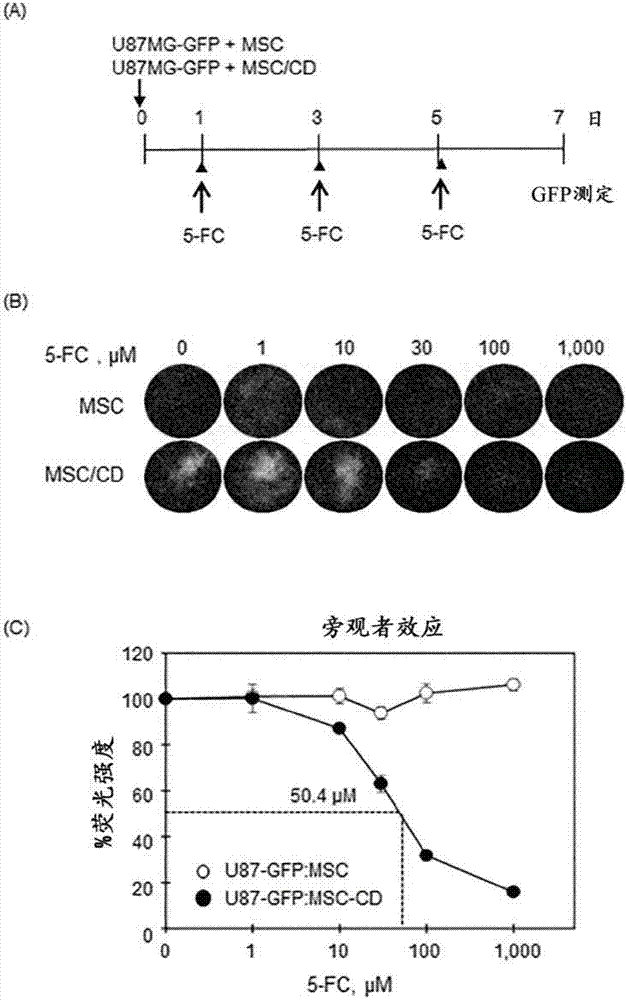
PendingCN107454844AGood tumor suppressor effectMaximize the effect of cancer treatmentOrganic active ingredientsNervous disorderCytosine deaminaseProliferative capacity
The present invention relates to a method for preparing cells for cancer treatment and a kit for cancer treatment comprising cells prepared by the method. The preparation method of the present invention can provide F cells, which, in spite of having no difference in the proliferative capacity compared with mesenchymal stem cells expressing cytosine deaminase, which are harvested and used immediately after the culture, exhibit a very excellent tumor suppressive effect through the treatment together with 5-FC and induce a remarkable synergistic effect exceeding an effect from combinative treatment with an existing anticancer drug in cases of a combinative treatment with another anticancer drug. Therefore, the present invention can be utilized for a kit for cancer treatment comprising such F cells, and thus can be favorably used to maximize the effect of existing cancer treatments.
Owner:CELL & BRAIN CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com