Mature type-1 polarized dendritic cells with enhanced IL-12 production and methods of serum-free production and use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
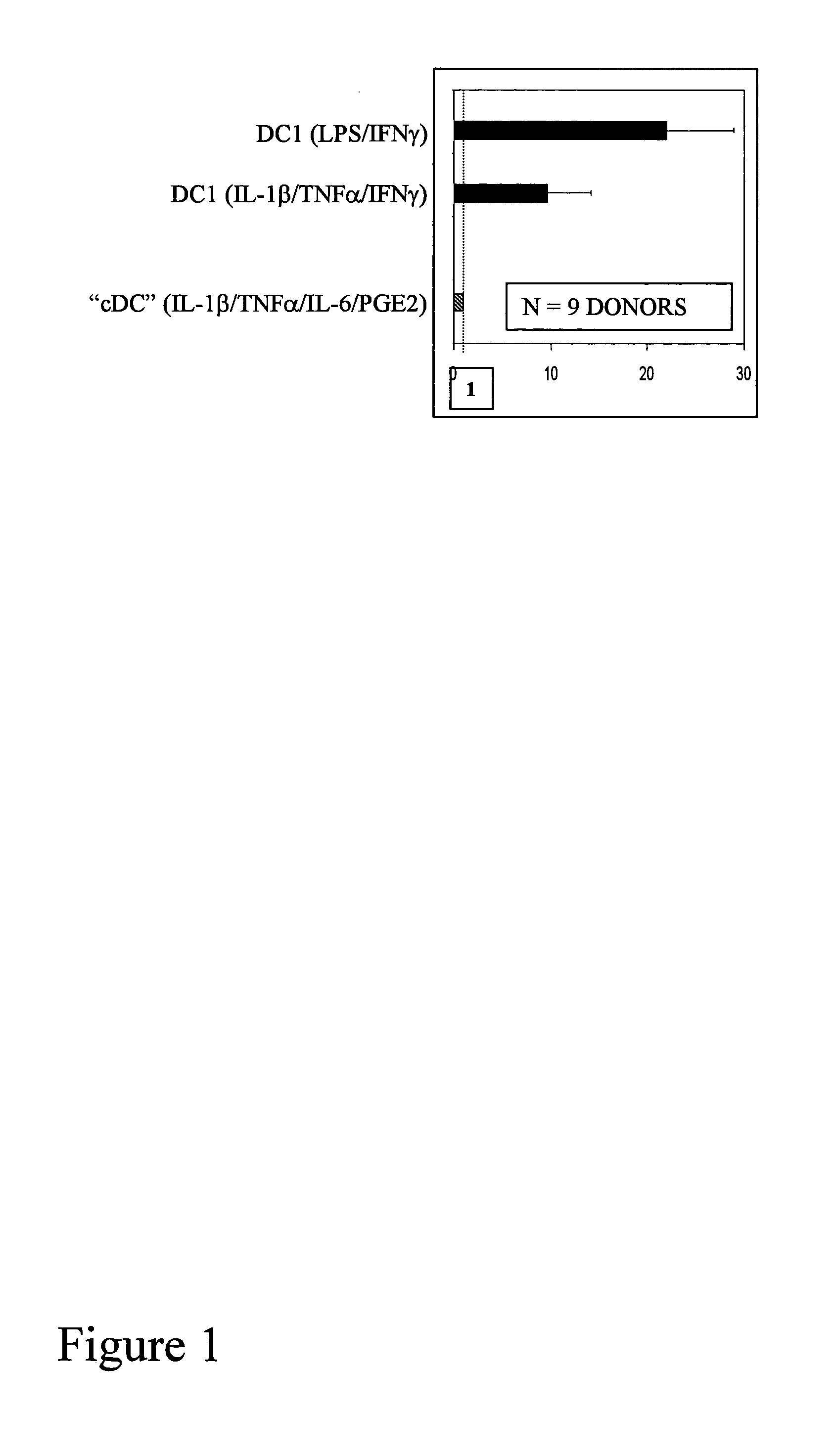
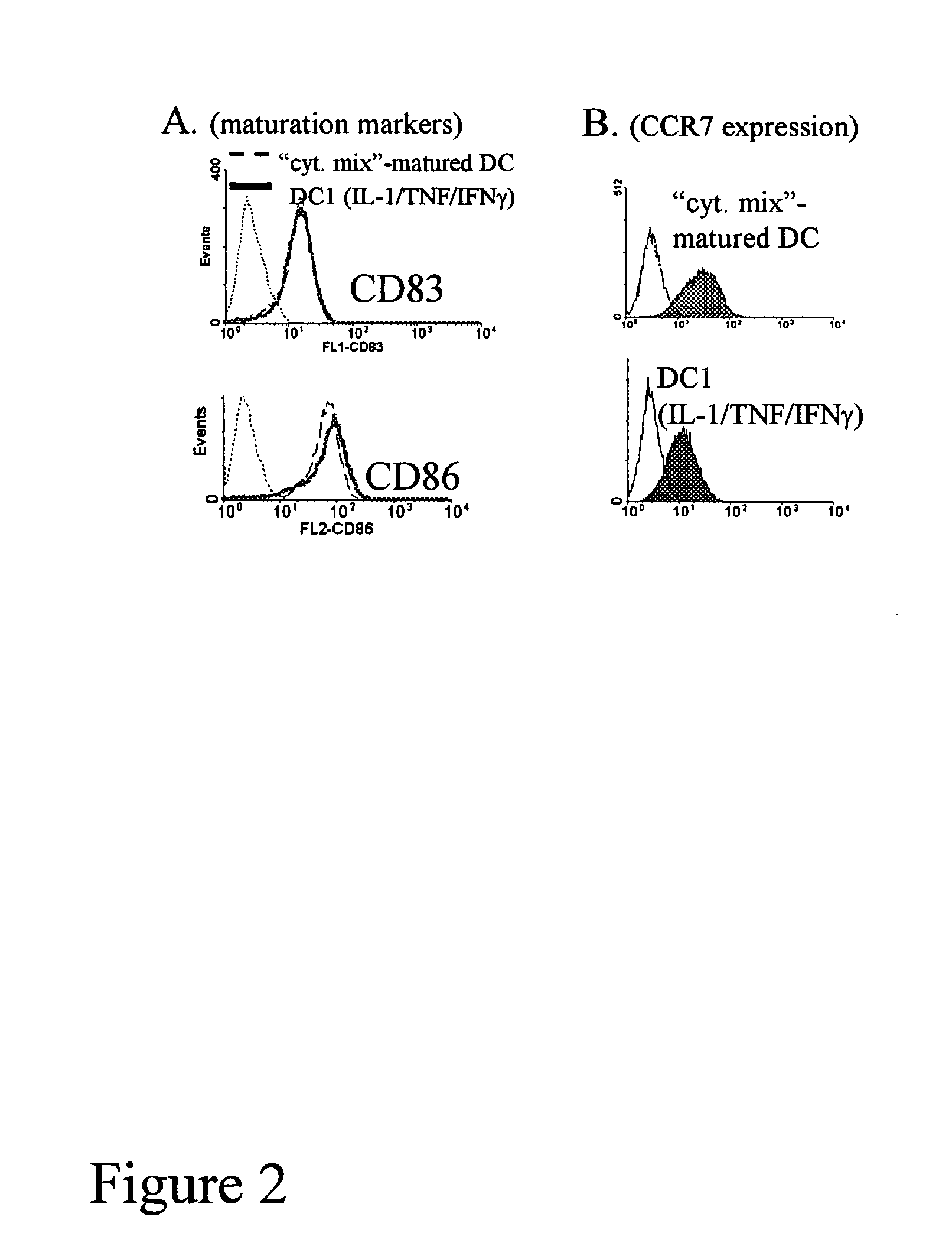
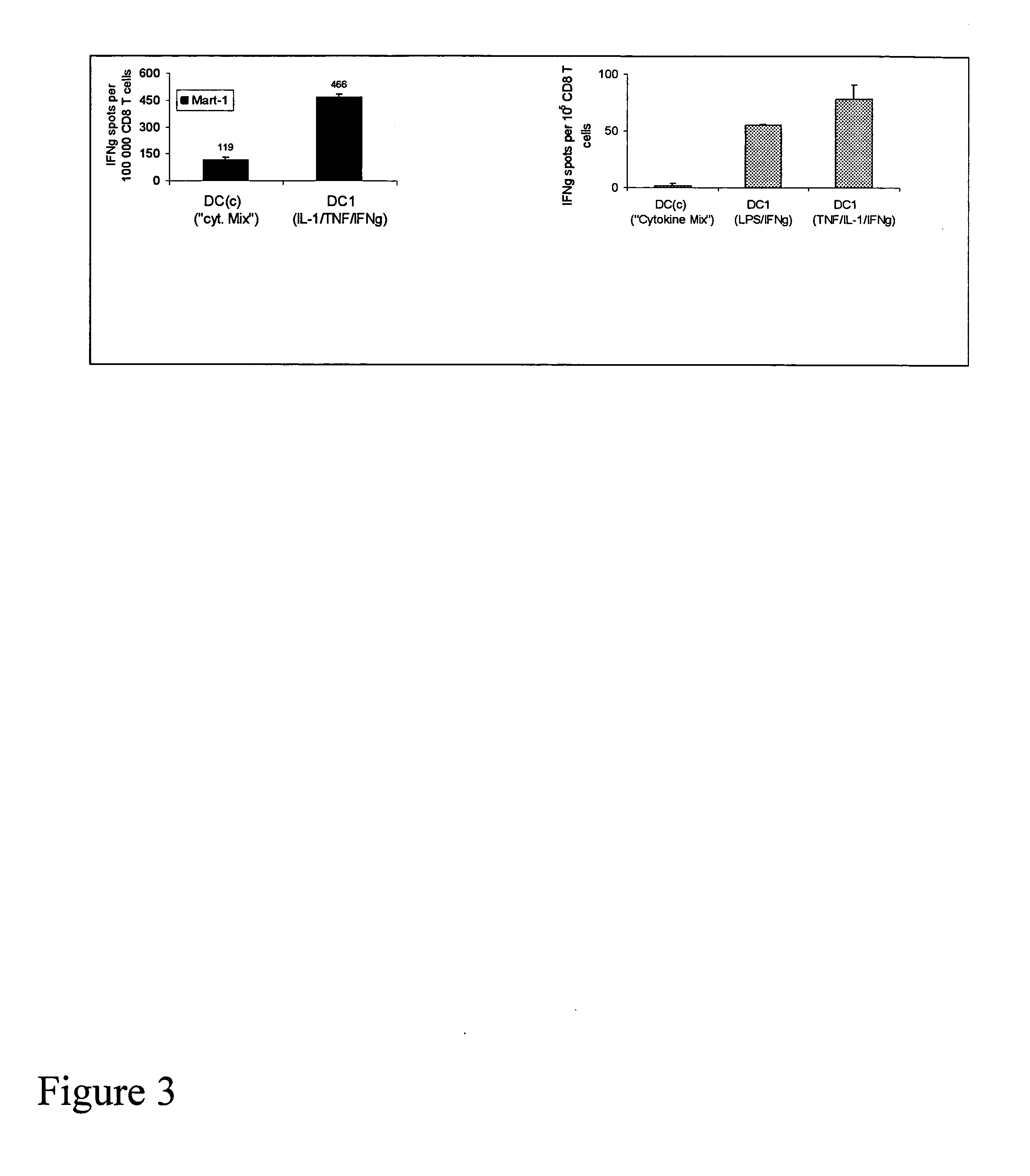
[0020] To boost the immunogenic capacity of DCs and their ability to induce high-intensity Th1 and CTL-mediated type-1 immune responses, the present invention combines within one DC type a fully-mature status and a high ability to produce high levels of IL-12p70. In contrast to current methodologies in which the final maturation of DCs induced by typical stimuli is associated with reduced ability to produce IL-12 (Kalinski et al., 1999, Langenkamp et al., 2000), the present invention provides for concomitant exposure of immature DC to a maturation-inducing stimulus and to IFNγ which results in a strong enhancement of the subsequent ability of mature DC to produce IL-12 and to induce Th1-dominated responses (Vieira et al., 2000, Mailliard et al., 2002), and more specifically the cancer-specific CTL responses.
[0021] Further, although current DC1-inducing protocols are ineffective in serum-free conditions, the present invention provides that IFNα, a member of type I interferon family,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com