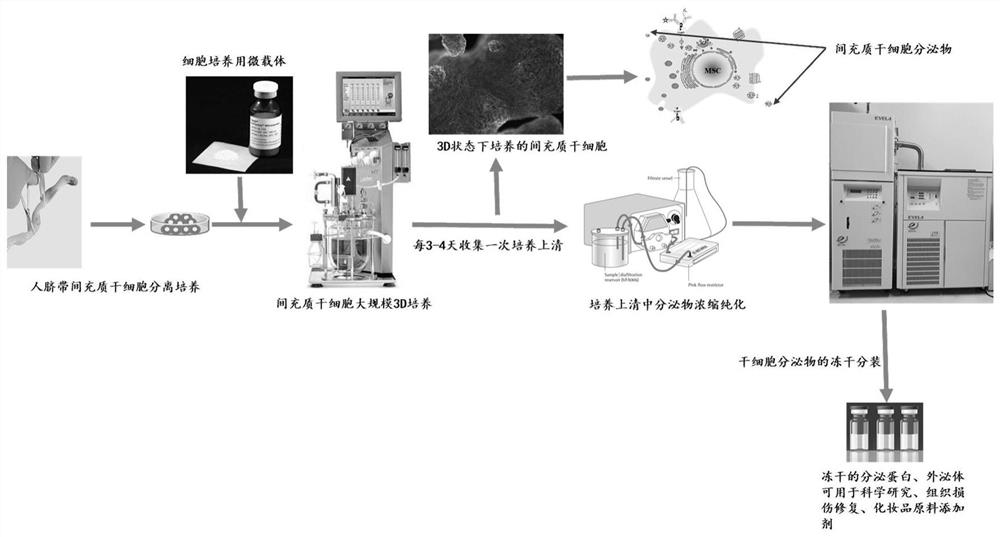
Culture method of mesenchymal stem cells
A culture method and mesenchymal stem cell technology, applied in the field of mesenchymal stem cell culture, can solve problems such as unknown specific components, inability to completely exclude heterologous viruses, and inability to grow cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] This embodiment provides a 2D culture method of mesenchymal stem cells, the formula of the serum-free medium used is as follows:
[0084] Each 1L medium consists of the following components:
[0085] IMDM 17.662g, L-alanyl-glutamine 2mM, chemical lipid 0.6v / v%, cholesterol 15mg, insulin 12.5mg, transferrin 25mg, recombinant human albumin 2g, hydrocortisone 500μg, dexamethasone 4μg, progesterone 5.66μg, putrescine 9mg, ascorbic acid 100mg, β-mercaptoethanol 75μM, soybean lecithin 10mg, zinc sulfate heptahydrate 2.5mg, lipoic acid 0.2mg, N-Acetyl-L-Cysteine 1mM, reduced glutathione Glycine 2mg, Taurine 5mg, Y-27632 5μM, Ethanolamine 2mg, b-FGF 20μg, EGF 20μg, PDGF-BB 10μg, IGF-1 15μg, TGF-β3 2μg, HGF 10μg, CTGF 2μg and VEGF 15μg; The amount is water for cell culture.
[0086] The configuration method of the serum-free medium is as follows:
[0087] Dissolve IMDM powder in cell culture water, stir well, filter with 0.22um filter membrane, add recombinant human insulin...
Embodiment 2
[0096] This example provides a 2D culture method for mesenchymal stem cells, the difference from Example 1 is that the formula of the serum-free medium used is as follows:
[0097] Each 1L medium consists of the following components:
[0098] IMDM 17.662g, L-alanyl-glutamine 2mM, chemical lipid 0.1v / v%, cholesterol 5mg, insulin 8mg, transferrin 10mg, recombinant human albumin 1g, hydrocortisone 0.1mg, dexamethasone 4μg, progesterone 1μg, putrescine 5mg, ascorbic acid 25mg, β-mercaptoethanol 50μM, soybean lecithin 2mg, zinc sulfate heptahydrate 1.25mg, lipoic acid 0.1mg, N-Acetyl-L-Cysteine 0.2mM, reduced glutathione Glycine 1mg, Taurine 2mg, Y-27632 2μM, Ethanolamine 1mg, b-FGF 5μg, EGF 5μg, PDGF-BB 2μg, IGF-1 10μg, TGF-β3 1μg, HGF 2μg, CTGF 1μg and VEGF 5μg; The amount is water for cell culture.
[0099] Its configuration method is the same as embodiment 1.
Embodiment 3
[0101] This example provides a 2D culture method for mesenchymal stem cells, the difference from Example 1 is that the formula of the serum-free medium used is as follows:
[0102] IMDM 17.662g, L-alanyl-glutamine 2mM, chemical lipid 1v / v%, cholesterol 30mg, insulin 25mg, transferrin 30mg, recombinant human albumin 5g, hydrocortisone 2mg, dexamethasone 20μg, Progesterone 10μg, putrescine 15mg, ascorbic acid 200mg, β-mercaptoethanol 100μM, soybean lecithin 20mg, zinc sulfate heptahydrate 2.5mg, lipoic acid 0.5mg, N-Acetyl-L-Cysteine 2mM, reduced glutathione 5mg , taurine 10mg, Y-27632 10μM, ethanolamine 5mg, b-FGF 40μg, EGF 50μg, PDGF-BB 20μg, IGF-1 40μg, TGF-β3 5μg, HGF 10μg, CTGF 5μg and VEGF 20μg; the balance is cell culture use water.
[0103] Its configuration method is the same as embodiment 1.
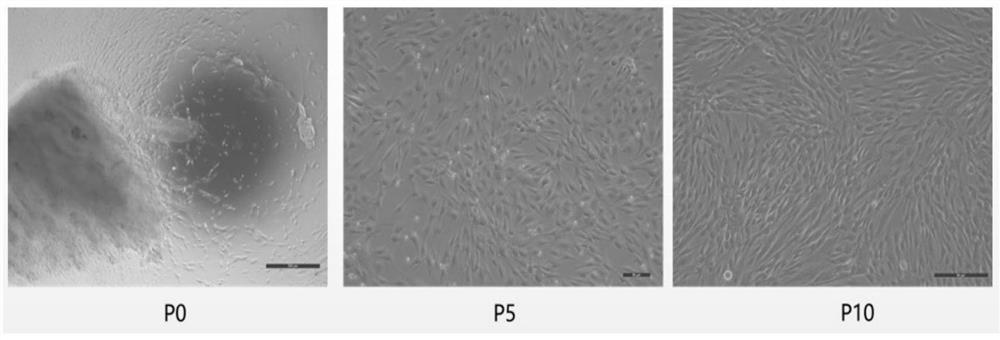
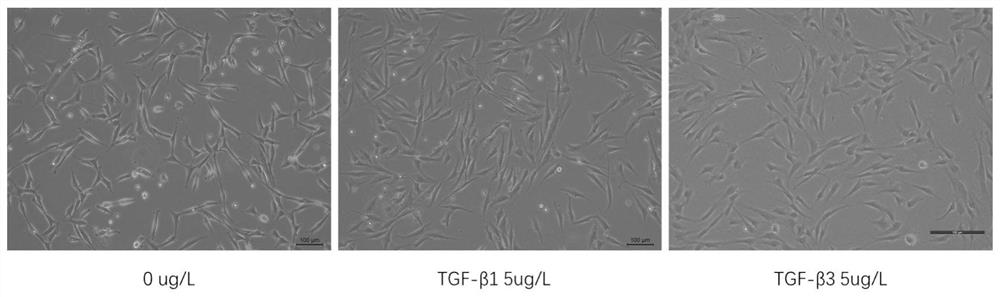
[0104] The same strain of P1 generation human umbilical cord mesenchymal stem cells was taken for testing, and three groups of repeated experiments were carried out in each o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com