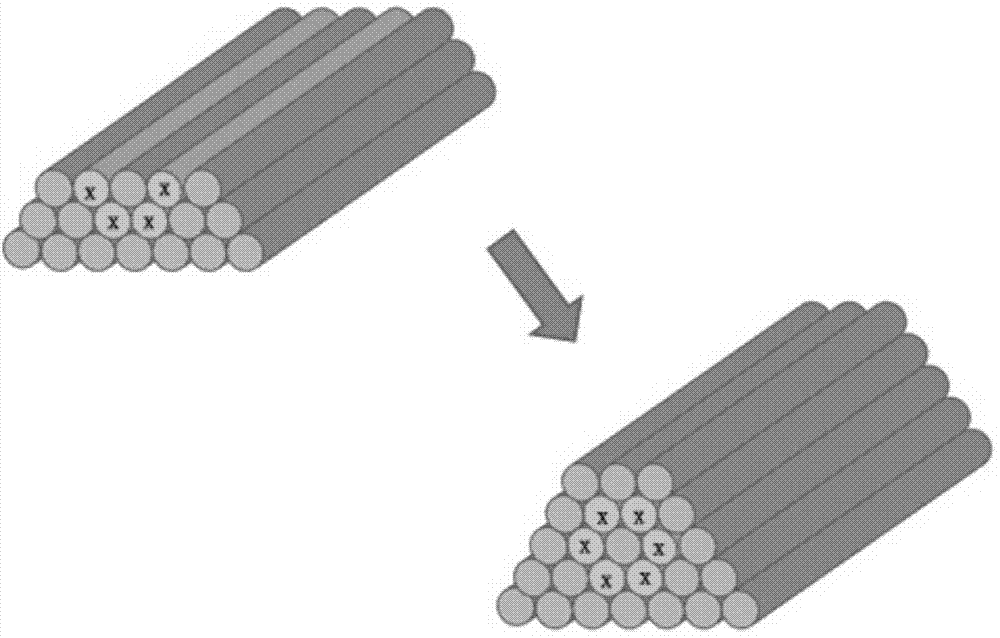
Method for preparing tissue engineering blood vessel based on 3D bioprinting technology
A tissue engineering and bioprinting technology, applied in the field of bioengineering, can solve the problems of low cell density, low cell compatibility and tissue compatibility of replacement blood vessels, insufficient mechanical strength, etc., achieve good biocompatibility, improve cell Compatibility and histocompatibility, the effect of overcoming the lack of mechanical strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Cell handling:
[0047] 1) Take out the culture dish containing human neonatal dermal fibroblasts (hNDFs) from the incubator, wash with phosphate buffer saline (PBS), and digest the cells with trypsin;
[0048] 2) The treated cells were resuspended in 4 mL of cell culture medium and placed in a 10 mL tissue culture flask;
[0049] 3) The flask is placed on a rotary shaker in a volume concentration of 5% CO 2 Environment, cultivated at 37°C for 1 hour, and centrifuged at 3500rpm;
[0050] 4) Use a 3D printer to extrude the cell column on a special, non-adhesive polytetrafluoroethylene or agarose base, and culture it overnight to mature the cell column.
[0051] Pluronic F127 (PF127) processing:
[0052]PF127 with 20%-30% W / W is used, wherein, W / W is: solute mass percent concentration (w / w) = solute mass / solution mass
[0053] Select liquid Pluronic F127 at a temperature of 4-5°C. When the material is sucked into the micropipette on the print head, it is immersed in P...
Embodiment 2
[0068] Cell handling:
[0069] 1) Take out the culture dish containing human neonatal dermal fibroblasts (hNDFs) from the incubator, wash with phosphate buffered saline (PBS), and digest the cells with trypsin;
[0070] 2) The treated cells were resuspended in 4 mL of cell culture medium and placed in a 10 mL tissue culture flask;
[0071] 3) The culture flask was placed on a rotary shaker at a volume concentration of 5% CO 2 Environment, culture at 37°C for 1 hour, centrifuge at 3500rpm;
[0072] 4) Use a 3D printer to extrude the cell column on a special, non-adhesive polytetrafluoroethylene or agarose base, and culture it overnight to mature the cell column;
[0073] Poly-N-isopropylacrylamide (PNIPAAm) treatment:
[0074] Select liquid poly-N-isopropylacrylamide, when the material is sucked into the micropipette on the print head, immerse in PBS to solidify the material, and continuously extruded strips of poly-N-isopropylacrylamide can be obtained;
[0075] 3D blood v...
Embodiment 3
[0088] Cell handling:
[0089] 1) Take out the culture dish containing human neonatal dermal fibroblasts (hNDFs) from the incubator, wash with phosphate buffered saline (PBS), and digest the cells with trypsin;
[0090] 2) The treated cells were resuspended in 4 mL of cell culture medium and placed in a 10 mL tissue culture flask;
[0091] 3) The culture flask was placed on a rotary shaker at a volume concentration of 5% CO 2 Environment, culture at 37°C for 1 hour, centrifuge at 3500rpm;
[0092] 4) Use a 3D printer to extrude the cell column on a special, non-adhesive polytetrafluoroethylene or agarose base, and culture it overnight to mature the cell column;
[0093] Methylcellulose (MC) treatment:
[0094] The content of methylcellulose (MC) is 12%-16%, and PBS is added to form the MC-water-salt system.
[0095] Select the above-mentioned liquid methylcellulose, when the material is sucked into the micropipette on the print head, immerse in PBS above 32°C to solidify t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Wall thickness | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com