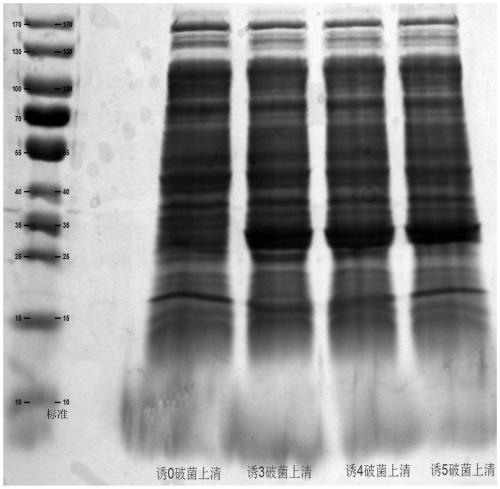
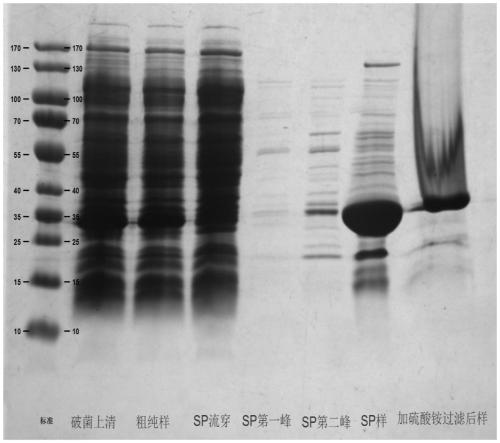
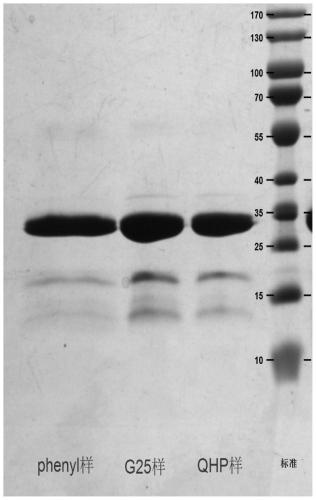
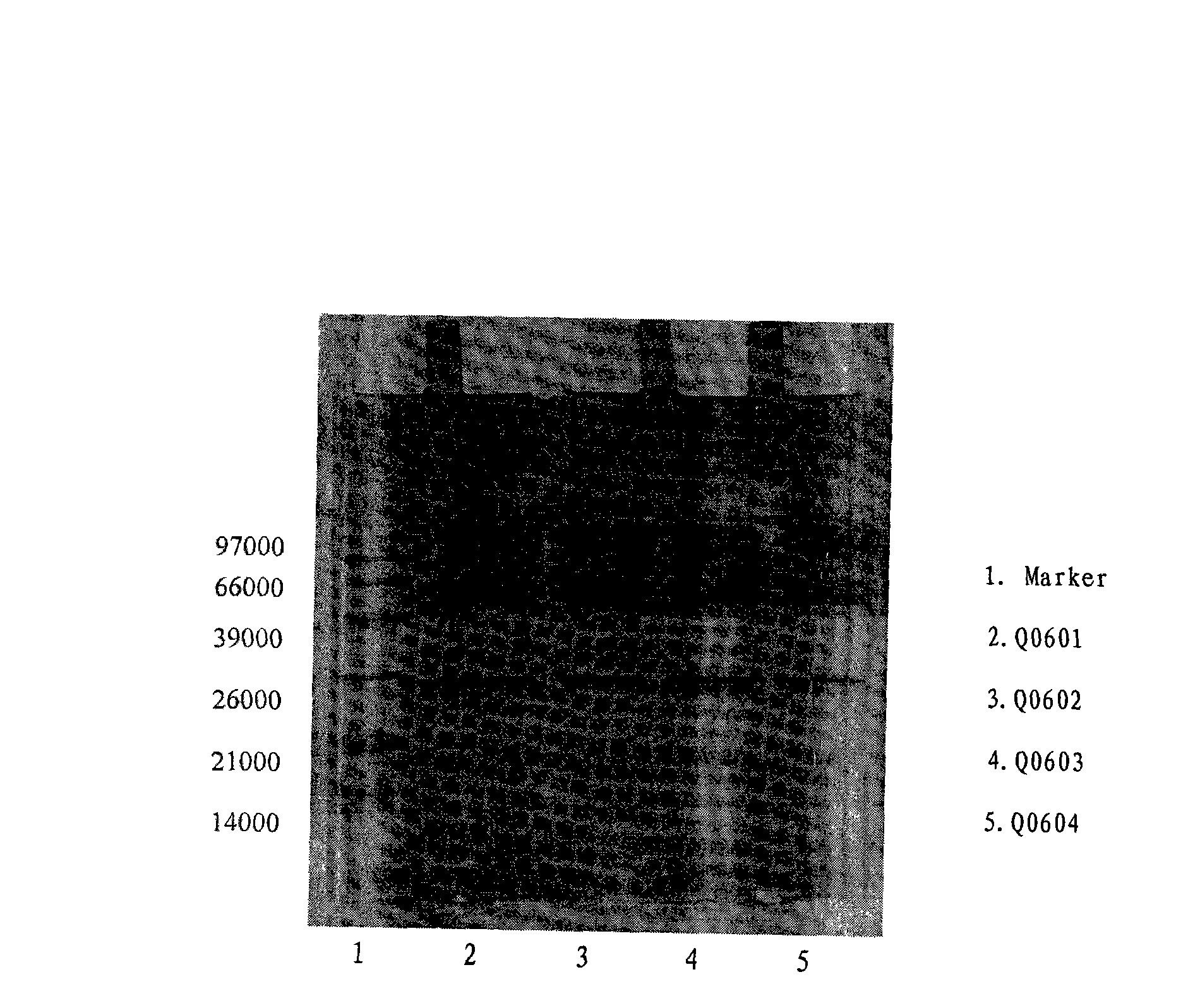
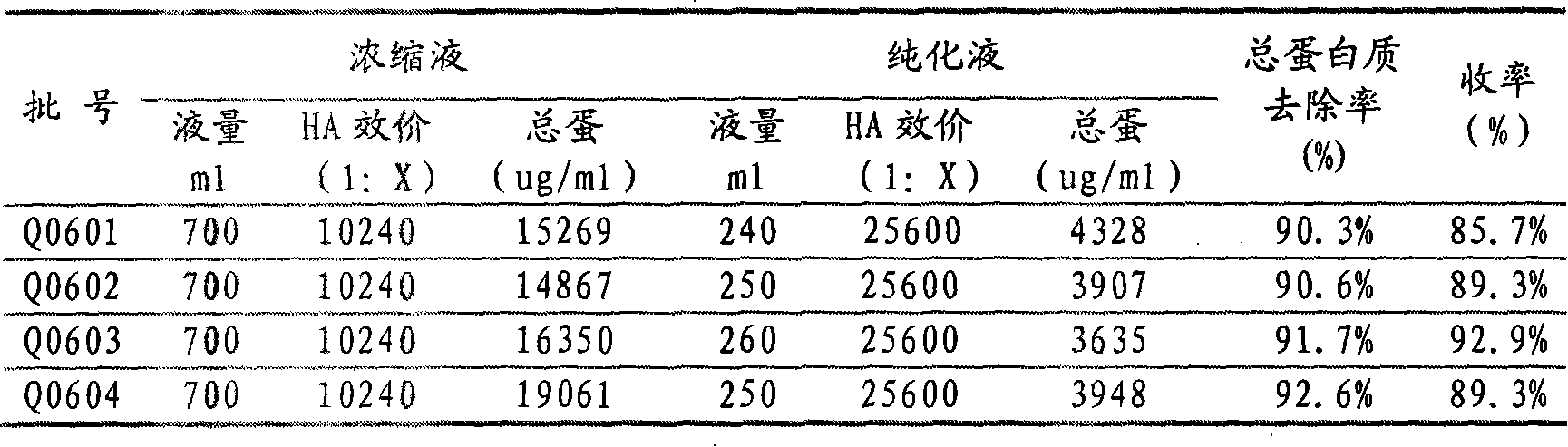
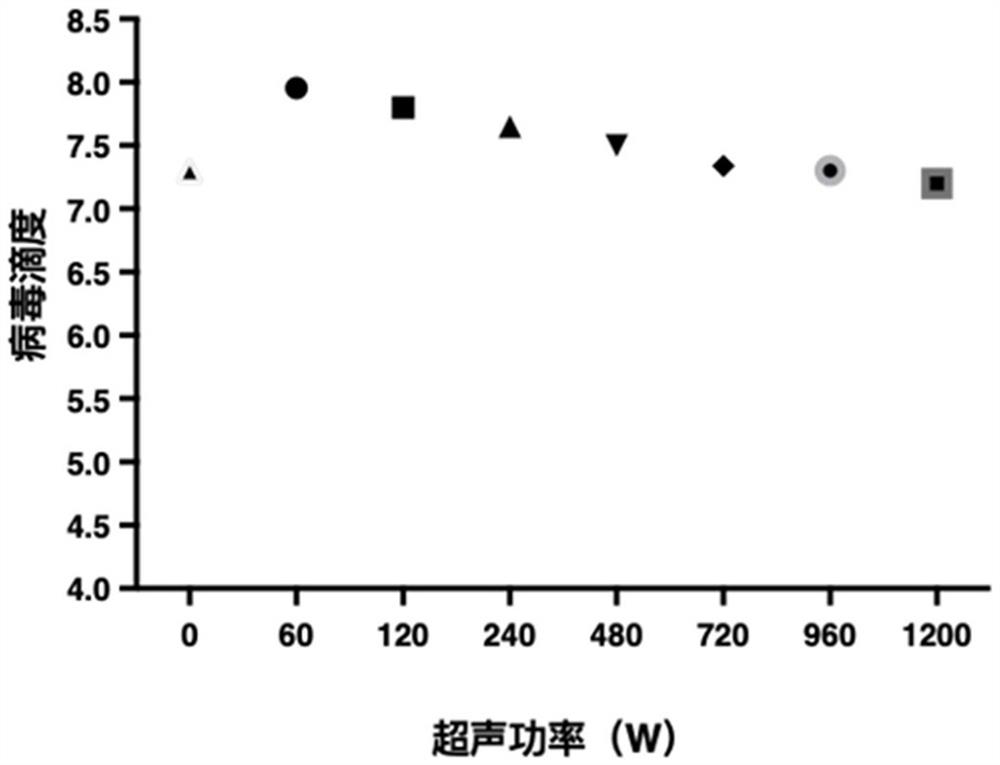
Patents
Literature
33 results about "Continuous flow centrifugation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
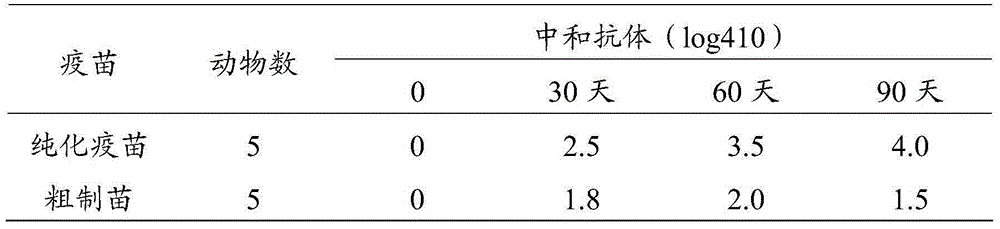
Method for preparing purified foot-and-mouth disease vaccine
InactiveCN103374547ARule out emergency responseReduced risk of contamination with exogenous agentsAntiviralsVertebrate cellsContinuous flow centrifugationSaccharum
The invention discloses a method for preparing a purified foot-and-mouth disease vaccine, a serum or animal-derived ingredient free culture medium and an application of the serum or animal-derived ingredient free culture medium to the preparation of the foot-and-mouth disease vaccine, belonging to the filed of biotechnology. The method for preparing the purified foot-and-mouth disease vaccine comprises the following steps of: culturing a foot-and-mouth disease virus by using the serum or animal-derived ingredient free culture medium, purifying an obtained virus solution to obtain a purified antigen, subjecting a cell strain BHK-21 or BSR to the multiple-generation acclimatization culture and the suspension culture by 300L of a microcarrier through the serum-free culture medium, inoculating the cell strain BHK-21 or BSR against the foot-and-mouth disease vaccine, stirring at the rotating speed of 30-50rpm, microfiltrating, ultrafiltrating, concentrating 50-200 times, carrying out chromatography with a Sephawse6FF molecular sieve or density gradient zonal centrifugation with a continuous flow, and inactivating with beta-propiolactone to obtain the serotype univalent or multivalent vaccine for cattle, sheep and pigs.
Owner:北京必威安泰科技有限公司 +1
Method for large-scale production of high-purity porcine pseudorabies virus
ActiveCN107254449ASolve the technical problems that are difficult to achieve large-scale production under high purityEfficient productionDsDNA virusesAntigenFiber
The invention belongs to the technical field of vaccines and in particular relates to a method for large-scale production of a high-purity porcine pseudorabies virus. The method comprises processes of continuous flow centrifugation, hollow fiber clarification filtering, ultrafiltration and concentration, and molecular sieve purification. The virus recycling rate is increased to the maximum extent, and the content of impurity proteins is reduced. A porcine pseudorabies virus concentrated solution and purified antigen produced by using the method are particularly applicable to vaccine preparation, and compared with a porcine pseudorabies virus inactivated vaccine produced according to the prior art, the high-purity porcine pseudorabies virus is relatively high in safety, relatively high in uniformity and relatively good in immune effect, and side reactions of vaccines are fundamentally reduced.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Method for large-scale production of porcine pseudorabies inactivated vaccine
ActiveCN107267466AHigh purityLess side effectsViral antigen ingredientsInactivation/attenuationMolecular sieveAdjuvant
The invention belongs to the technical field of vaccines and relates to a method for large-scale production of a porcine pseudorabies inactivated vaccine. The method comprises preparing a virus solution of porcine pseudorabies (XF-1 strain), carrying out continuous flow centrifugation, hollow fiber column clarification filtration, hollow fiber column ultrafiltration concentration and Sepharose 4FF molecular sieve gel chromatography purification treatment to obtain purified porcine pseudorabies viruses, adding a formaldehyde solution having a final concentration of 0.4% (v / v) into the purified porcine pseudorabies viruses, carrying out inactivation at 37 DEG C for 48h, and carrying out emulsification with a 201 adjuvant to obtain the porcine pseudorabies inactivated vaccine. The porcine pseudorabies inactivated vaccine can well prevent highly pathogenic mutant pseudorabies prevailing in the market.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Perfusion bioreactor
InactiveUS20120100576A1Improve production yieldMaintaining and even improving quality of productBioreactor/fermenter combinationsFungiPerfusion bioreactorCulture cell
The present invention pertains to a system for culturing cells comprising a culturing bag and a continuous flow centrifuge wherein the cells are continuously separated from the supernatant and are recycled into the culturing bag. Further provided are methods for culturing cells and for producing a biological substance using the device for culturing cells, and the use of a bag for culturing cells in said device or said methods. In particular, a perfusion system for culturing cells is provided wherein the wave technology for culturing cells is combined with continuous flow centrifugation for separating the medium from the cells.
Owner:GLYCOTOPE GMBH
Large-scale preparation method of recombinant staphylococcus aureus vaccine
PendingCN110343633AEasy to prepareRealize industrial scale productionAntibacterial agentsBacteriaContinuous flow centrifugationStaphylococcus aureus
The invention discloses a large-scale preparation method of a recombinant staphylococcus aureus vaccine. The method comprises the following steps: opening a protein working seed lot, inoculating the protein working seed lot strain in a conical flask, inoculating first-generation production strain in a seeding tank, performing continuous second-generation cultivation, inoculating the second-generation production strain in a fermentation tank, and performing continuous third-generation cultivation; centrifugally removing supernate from a bacterial solution through a continuous flow after fermentation is completed; and performing bacterium body dissolving and bacterium disruption; performing combination, washing the mixture after combination is completed, performing digestion, purifying the protein digestion solution by adopting a chromatographic column and performing desalination, endotoxin removing, sterile filtration and storage. The preparation method is simple and can be used for preparing recombinant staphylococcus aureus vaccine in a large scale.
Owner:CHENGDU OLYMVAX BIOPHARM
Purification technology applicable to mass production of human-used avian influenza vaccine
The invention belongs to the field of biotechnology and relates to the purification technology for avian influenza viruses, which is applicable to the mass production of human-used avian influenza vaccines. The purification technology is characterized in that: the avian influenza viruses are inoculated on a 9 to 11 days chicken embryo; the influenza viruses are cultured for 68 to 72 hours; and the allantoic fluid of the chicken embryo is sequentially collected, coarsely filtered, centrifugated with a continuous flow, ultrafiltered, condensed, centrifugated in a sucrose density gradient to be purified. The technology has the advantages of simple operation, small difference between batches, stable quality, high yield, low ovalbumin content and the like.
Owner:DALIAN ALEPH BIOMEDICAL
Method for absorbing and separating human prothrombin complex by utilizing expansion bed
InactiveCN101838304AAdsorption separation stabilityEfficient Adsorption SeparationPeptide preparation methodsContinuous flow centrifugationPROTHROMBIN COMPLEX
The invention relates to a method for absorbing and separating a human prothrombin complex by utilizing an expansion bed, comprising the following steps: (a) separating cryoprecitation from melted refrigerated plasma by using a refrigeration type continuous flow centrifuge, and collecting supernate as a raw material; (b) carrying out stable expansion on an expansion bed by using a buffer solution A, wherein the buffer solution A is 0.05mol / L1tris-citric acid with the PH value of 7.0; (c) rapidly switching into a feed solution after the expansion bed is in stable balance, stopping feeding and rapidly switching into the buffer solution A when breakthrough points reaches 5-10%, and washing in an expanded mode; and (d) switching into buffer solutions of 0.4mol / L NaCl and 1.6mol / L NaCl after washing, carrying out gradient elution in a stationary bed mode, and collecting the protein elution peak of the buffer solution of 1.6mol / L NaCl to obtain a prothrombin complex crude product.
Owner:成都英德生物工程有限公司
High-capacity centrifugal machine rotor and continuous flow centrifugal machine
InactiveCN103252293AEasy to collectImprove centrifugation efficiencyCentrifugesContinuous flow centrifugationLiquid state
The invention discloses a high-capacity centrifugal machine rotor which comprises a rotor body. The rotor body is a hollow trapezoid cylinder body, wherein a material inlet and outlet is formed in the center of the top portion, a groove which sinks inwards is formed in the central position of the bottom portion of the trapezoid cylinder body, and a shaft hole is formed in the bottom portion of the groove. The invention further discloses a continuous flow centrifugal machine which comprises the high-capacity centrifugal rotor. The continuous flow centrifugal machine comprises a centrifugal machine main shaft, a liquid inlet pipe and a liquid outlet pipe, wherein the liquid inlet pipe and the liquid outlet pipe stretch into the inner portion of the rotor body through the material inlet and outlet, and the top end of the centrifugal machine main shaft penetrates through the shaft hole. The rotor body is erected and fixed on the centrifugal machine main shaft through a rotary shaft nut and a gasket corresponding to the rotary shaft nut so that the rotor body can rotate as the centrifugal machine main shaft rotates. Through the high-capacity centrifugal machine rotor and the continuous flow centrifugal machine, a large amount of mixed liquid can be centrifuged continuously, sedimentary solid-state substances can be collected conveniently, separation of liquid-state / solid-state substances in the mixed liquid can be completed at one time, centrifugal separation efficiency is improved greatly and centrifugal operating time is shortened.
Owner:苏州杰诺曼博生物科技有限公司
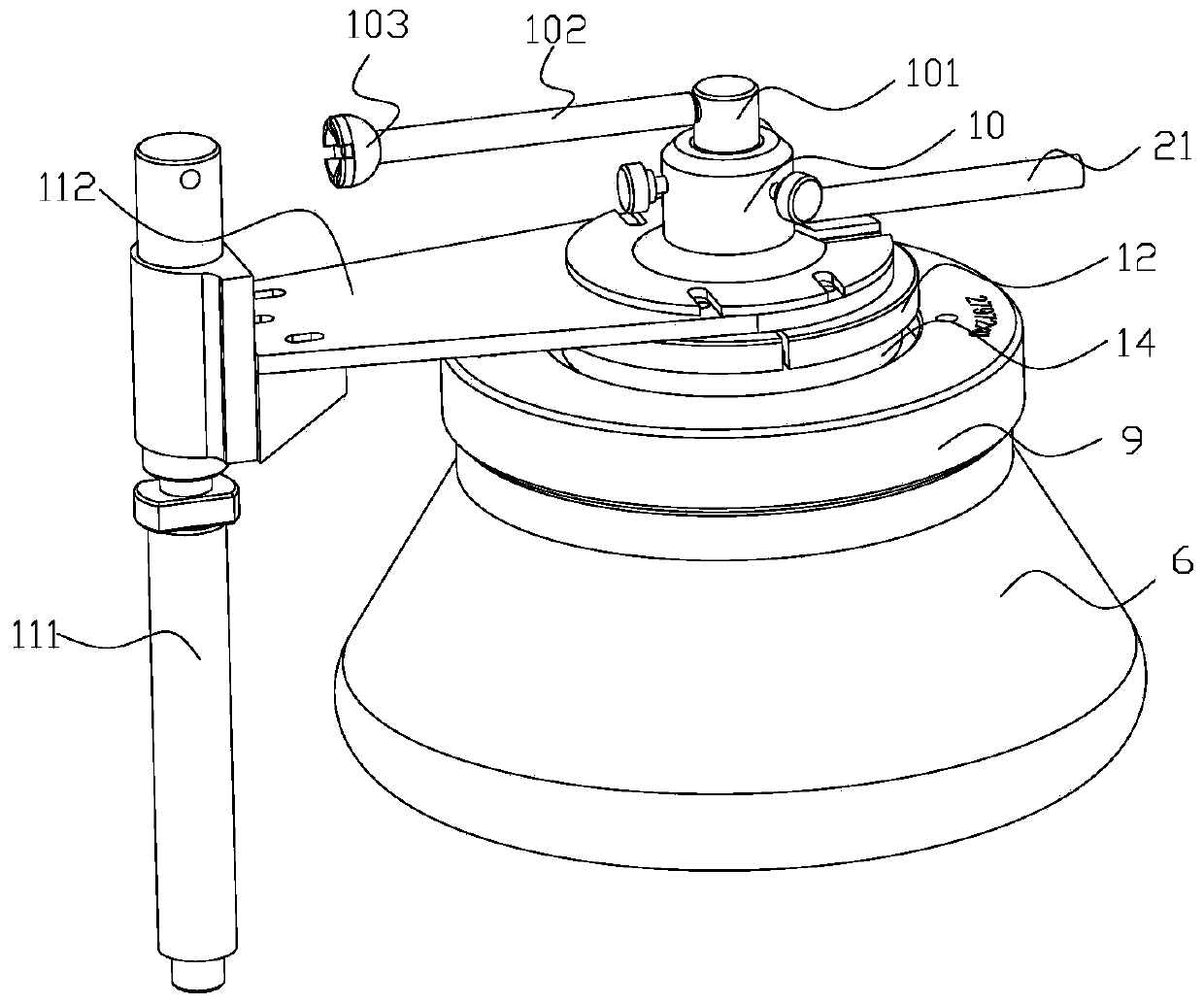
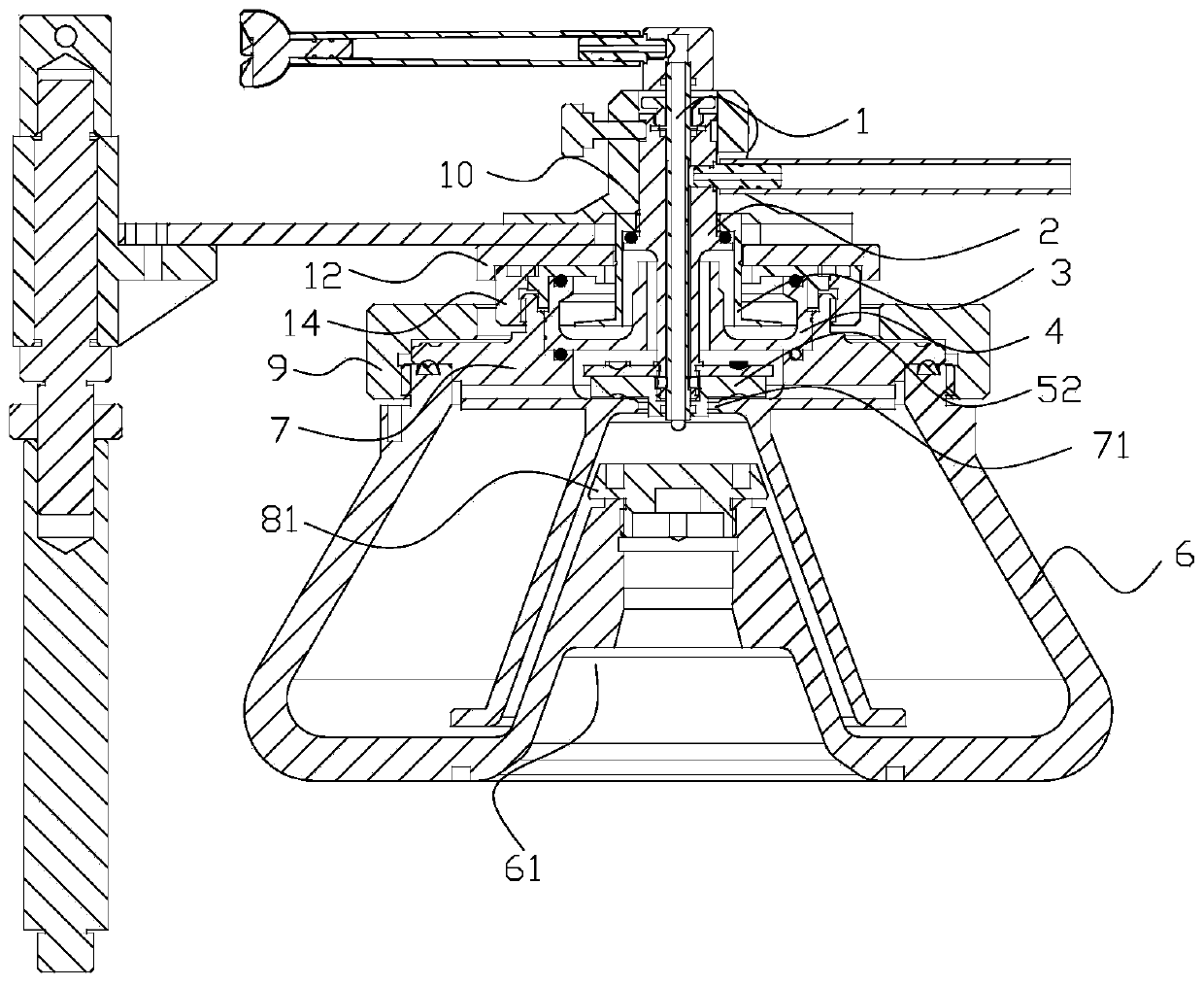
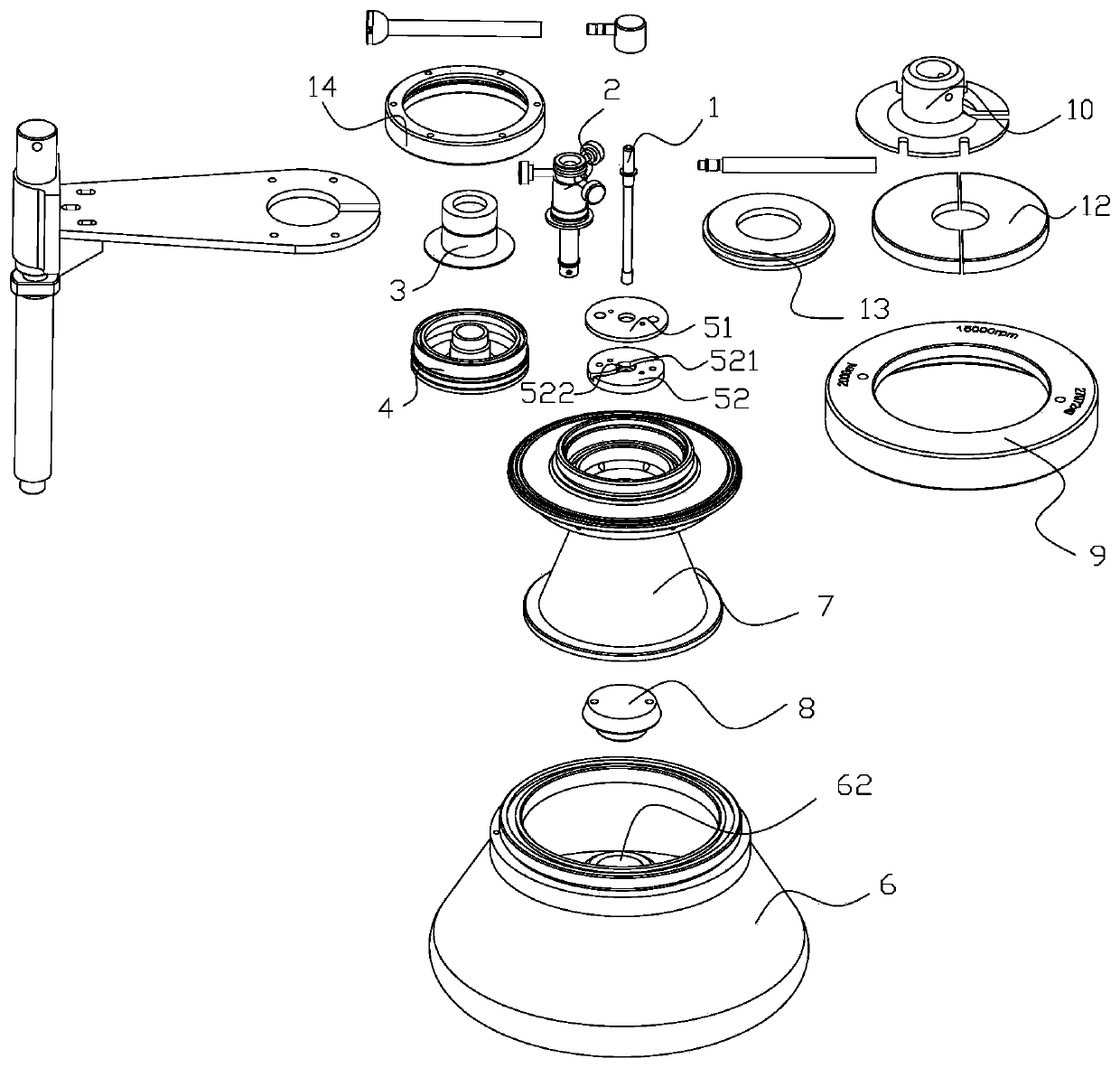
Continuous flow centrifuge rotor device and centrifuge
InactiveCN109772600ALarge capacityHigh speedCentrifugesCentrifuge rotorContinuous flow centrifugation
The invention relates to the technical field of centrifugal, in particular to a sterile continuous flow centrifuge rotor device and centrifuge equipment. The sterile continuous flow centrifuge rotor comprises a sample feeding pipe, a drain tube seat, an isolation sleeve, a sealing cover, a centripetal pump assembly, a rotor body, a rotor core, a transfer pin mounting block and a rotor cover, the drain tube seat is arranged on the sample feeding tube in a sleeving mode, the isolation sleeve is arranged at the lower part of the drain tube seat in a sleeving mode, the sealing cover is arranged atthe lower part of the isolation sleeve in a sleeving mode, the centripetal pump assembly comprises a centripetal pump cover body and a centripetal pump body, the upper part of the rotor core is arranged on the sealing cover in a sleeving mode, the rotor body is arranged on the rotor core in a sleeving mode, and the rotor cover is arranged at the upper part of the rotor core in a sleeving mode. The continuous flow centrifuge rotor device has the beneficial effects that the device is sterile so as to prevent the separated material from being polluted by the external bacteria, the capacity is high, the rotating speed and relative centrifugal force are high, the centrifugal effect is high, a liquid inlet and drain device is a static assembly, the service life of the liquid inlet and drain device is prolonged, the liquid inlet, centrifugation, and the draining are conducted continuously and simultaneously, and the efficient preparation is achieved.
Owner:王加皮
Method for extracting bio-polypeptides
InactiveCN106432407AIncrease productionHigh activityPeptide preparation methodsContinuous flow centrifugationIce water
The invention provides a method for extracting bio-polypeptides. The method comprises the following steps: removing fat and external envelops from brains, livers, spleens or thymus glands of fresh seafoods (oyster, shrimp flesh, crab flesh, sea cucumber and the like) and healthy animals, and grinding with a meat grinder; weighing, and homogenizing at high speed; treating the homogenized liquid in microwave for 15-30 minutes by adopting a continuous flow centrifuge centrifuging technology / gauze coarse filtering treatment, quickly standing in ice water at 4 DEG C, and performing ultrasonic treatment for 30-60 minutes; and performing ultrafiltration by utilizing a lateral ultrafiltration membrane having a molecular weight cut-off of 1000-12500 dlotons, and freezing and drying the ultrafiltration liquid to obtain the bio-polypeptide dry powder. The method can be used for increasing the bio-polypeptide yield to 230-450 percent, so that the yield of extracting bio-polypeptides from animal organs can be improved.
Owner:广东美利奥生物科技有限公司
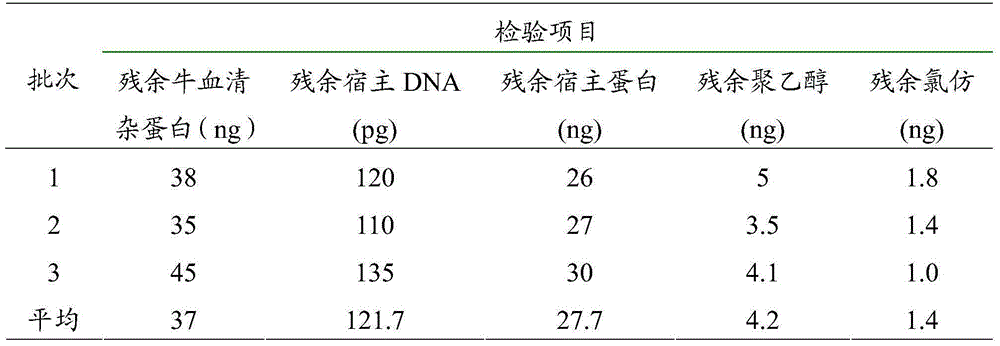
Method for industrially producing new born calf purified serum
InactiveCN110066762ANot lostGood removal effectVertebrate cellsArtificial cell constructsLipid formationElisa kit
The invention relates to a method for animal serum purification. New born calf raw material serum which is obtained after beestings is fed and contains a large amount of lipid, endotoxin, hemoglobin and other impurities is preliminarily filtered to remove fibrous protein; the serum is separated into an interception part (a macromolecule compound) and a dialysis part (free molecules) through an ultrafiltration column with the molecular weight cutoff being 100-300 kd; a solid phase deproteinization hydration shell medium is added into the interception part so as to promote lipid (containing chyle particles and lipoprotein), immune globulin, endotoxin, hemoglobin and other impurities to be polymerized into flocks, and continuous flow centrifuging and clarification filtering are performed to obtain clear liquid; the clear liquid and the ultrafiltration dialysis part are combined, and after degerming, new born calf purified serum is manufactured. By adopting the method, the new born calf serum which is obtained after beestings is fed and contains excessive endotoxin and hemoglobin can be processed into serum meeting the quality requirement of a cell culture medium for vaccines and an ELISA kit.
Owner:范清春
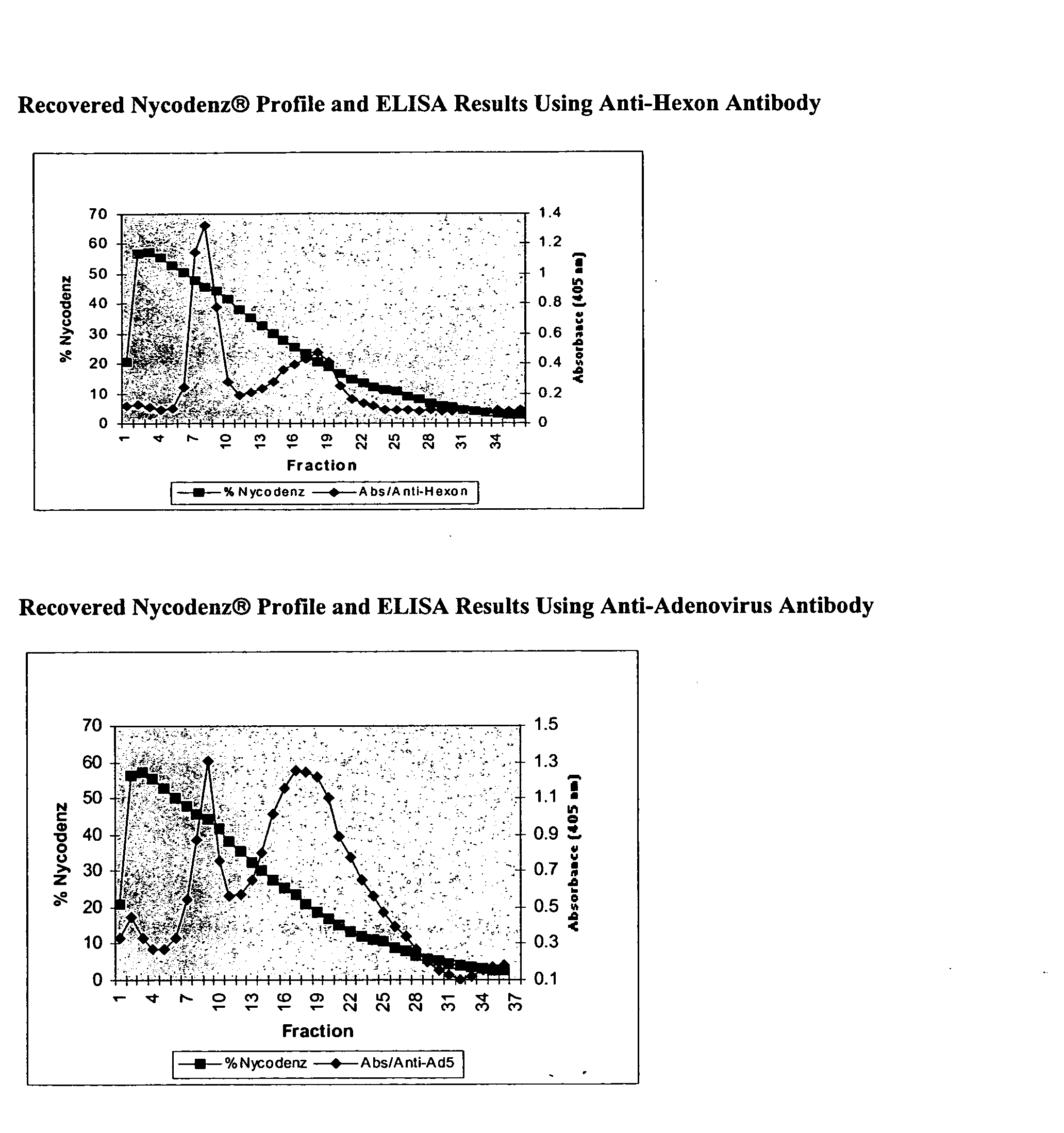
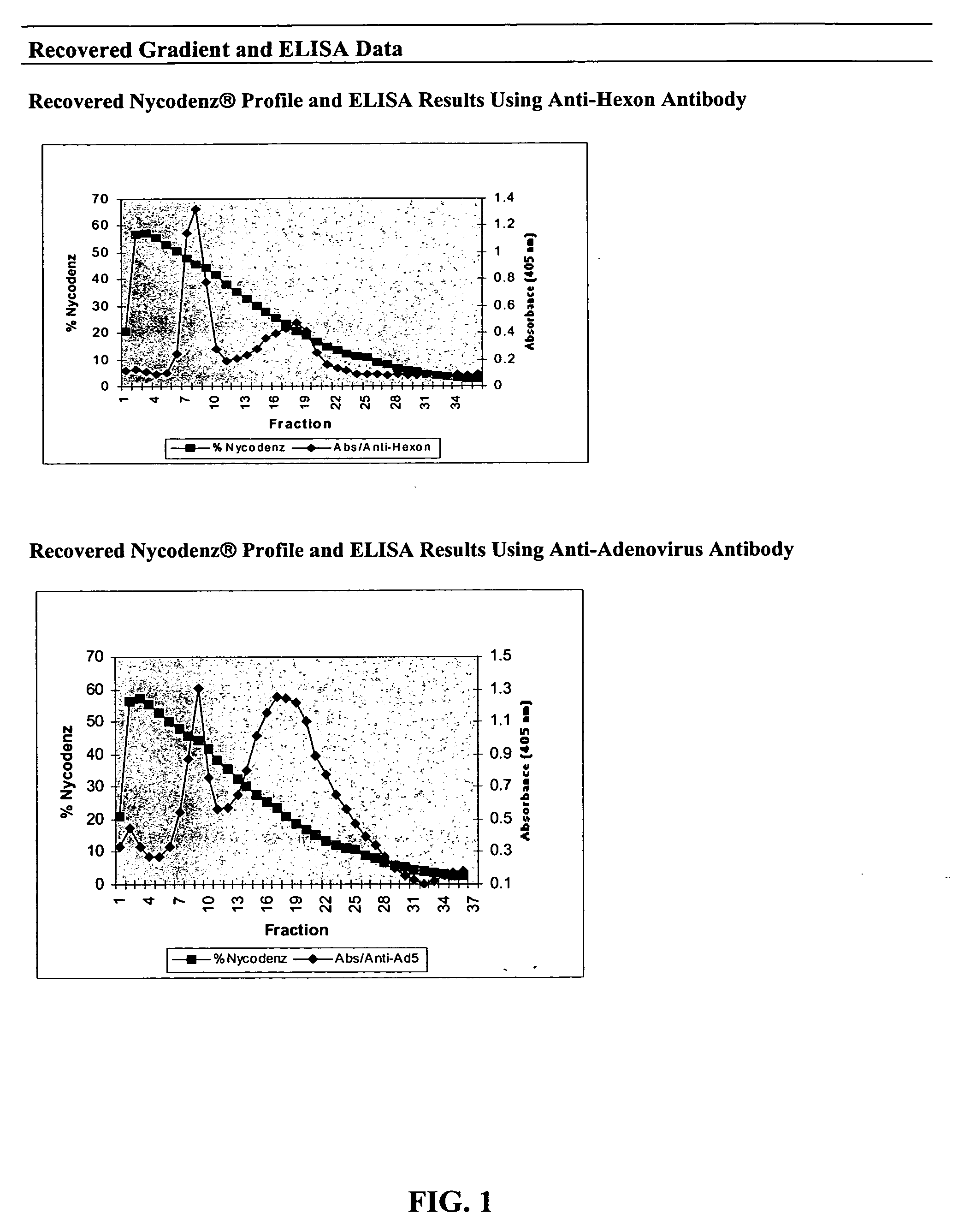
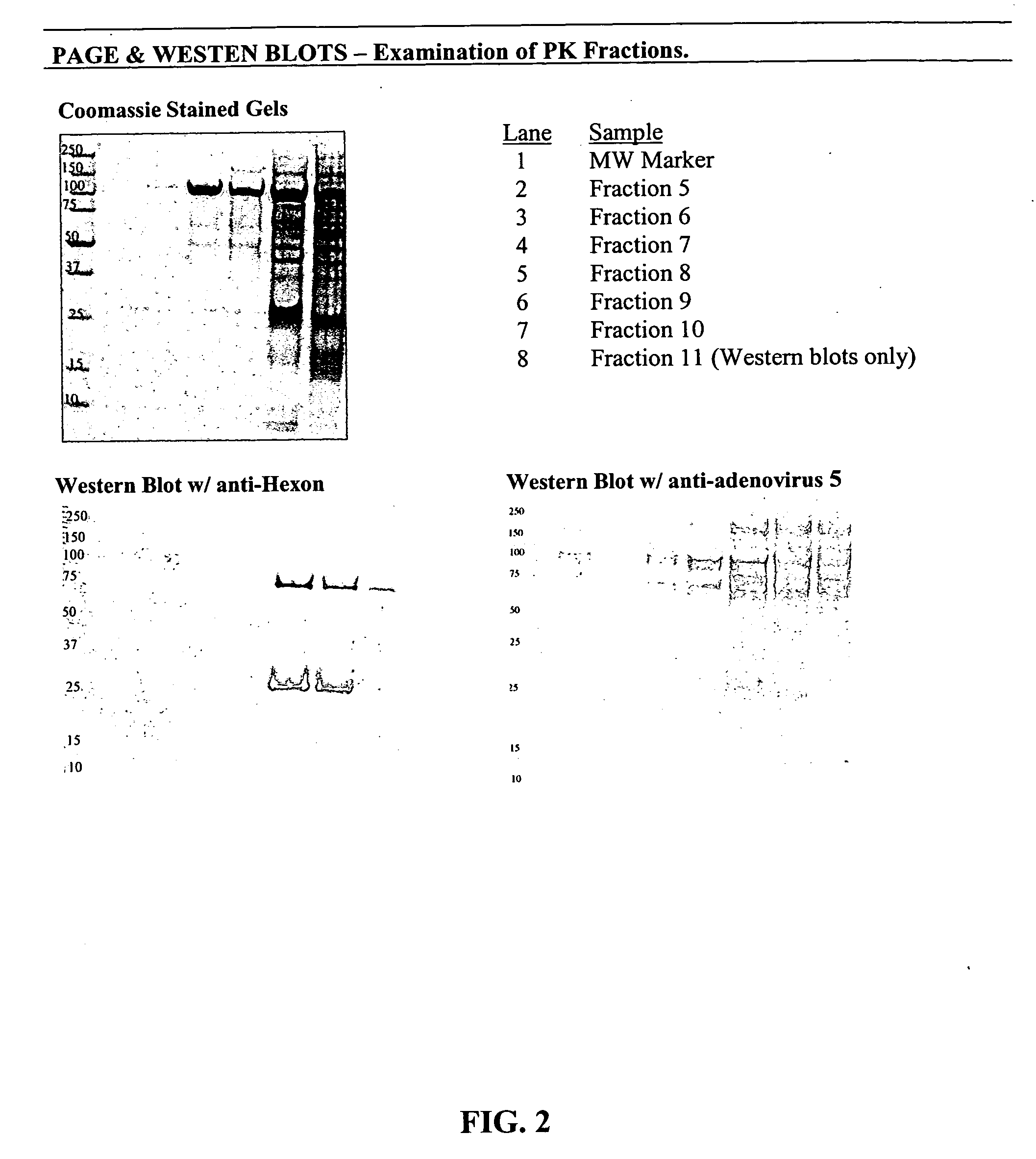
Processes and compositions for adenovirus purification using continuous flow centrifugation
InactiveUS20050189305A1High yieldReduce flow rateWater/sewage treatment by centrifugal separationMicrobiological testing/measurementContinuous flow centrifugationContinuous flow
Owner:FORRESTER KATHY
A kind of preparation method of foot-and-mouth disease purified vaccine
InactiveCN103374547BRule out emergency responseReduced risk of contamination with exogenous agentsAntiviralsVertebrate cellsSucroseContinuous flow centrifugation
Owner:北京必威安泰科技有限公司 +1
Process method for preparing biological product by using continuous flow centrifuge
ActiveCN114107051AReduce stepsImprove airtightnessBioreactor/fermenter combinationsBiological substance pretreatmentsContinuous flow centrifugationCell factory
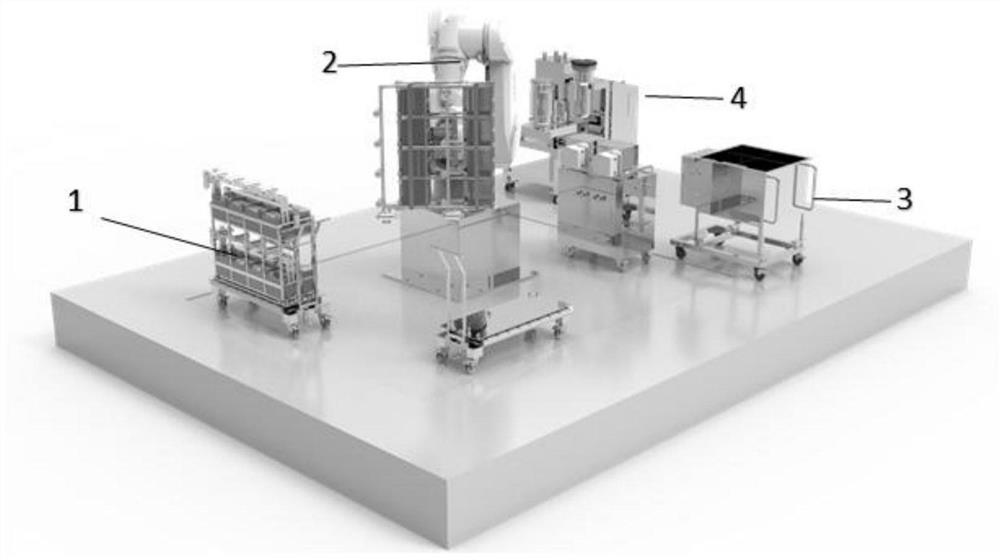
The invention discloses a process method for preparing a biological product by using a continuous flow centrifuge. The process method comprises the following steps: step 1, moving a cell factory framework to a working position of a six-axis sterile robot through a transfer trolley; 2, the cell factory framework is grabbed and placed through a six-axis sterile robot; step 3, adding liquid into the cell factory, discharging the liquid outwards from the cell factory, shaking, and stopping the action; and 4, preparing a culture stock solution through a one-time stirring system in combination with a continuous flow centrifuge. The robot is combined with the continuous flow centrifugal machine for use, operation flexibility is high, and the automation degree is high.
Owner:CHANGCHUN KEYGEN BIOLOGICAL PROD
Method for reducing cell protein and DNA (Deoxyribose Nucleic Acid) residues in rabies virus product
ActiveCN114645024ASsRNA viruses negative-senseViral antigen ingredientsContinuous flow centrifugationUltrafiltration
The invention provides a method for reducing cell protein and DNA residues in a rabies virus product. The method comprises the following steps: continuously performing circulating centrifugation on virus supernate in a tank by using a continuous flow centrifuge in a bioreactor virus culture stage, then perfusing the virus supernate into a bioreactor culture system, and simultaneously harvesting supernate in the tank and filtering the supernate through a proper filtering device. After the method is adopted for treatment, protein residues and DNA residues of virus production cells in the virus harvesting liquid are remarkably reduced, and the protein residues of Vero cells are detected to be far lower than the existing technical standard through ultrafiltration concentration and column chromatography purification of the virus liquid and preparation of the freeze-dried vaccine. Meanwhile, the method effectively reduces the downstream purification cost, effectively ensures that no residues of other substances such as nuclease exist in the virus product, and is suitable for large-scale production.
Owner:SHANGHAI RONGSHENG BIOLOGICAL PHARM CO LTD
Hospital sewage treatment method
InactiveCN113024014ASolve pollutionEfficient removalWater/sewage treatment by centrifugal separationMultistage water/sewage treatmentMicroorganismContinuous flow centrifugation
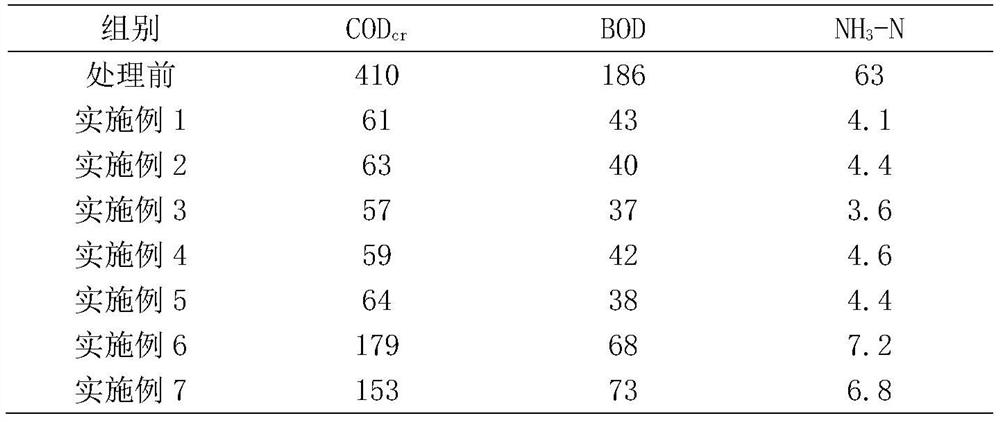
The invention belongs to the field of sewage treatment, and particularly relates to a hospital sewage treatment method which comprises the following steps: S1, sewage sterilization: after sewage generated by a hospital is collected, the sewage continuously passes through a tubular sterilization machine to be sterilized, and sterilized wastewater is obtained; S2, microbiological treatment: the sterilized wastewater is cooled, microorganisms are added into the cooled wastewater, and treating is conducted for 2-5 days, wherein the addition amount of the microorganisms is 1-6% of the weight of the wastewater; S3, centrifugal separation: centrifugal separation is conducted on the wastewater subjected to microbiological treatment in the step S2 by using a continuous flow centrifuge, clear liquid after centrifugation is discharged into a flocculation basin, and thalli are killed in a centralized manner; and S4, flocculation treatment: a flocculant is added into the clear liquid discharged into the flocculation basin, stirring is conducted for 30-50 minutes, standing is conducted for 60-120 minutes, and liquid supernatant is directly discharged, wherein the adding amount of the flocculating agent is 1-10% of the weight of the clear liquid. According to the method, CODcr (Chemical Oxygen Demand), BOD (Biochemical Oxygen Demand) and NH3-N in the hospital sewage are effectively removed.
Owner:云南和泽环保科技有限公司
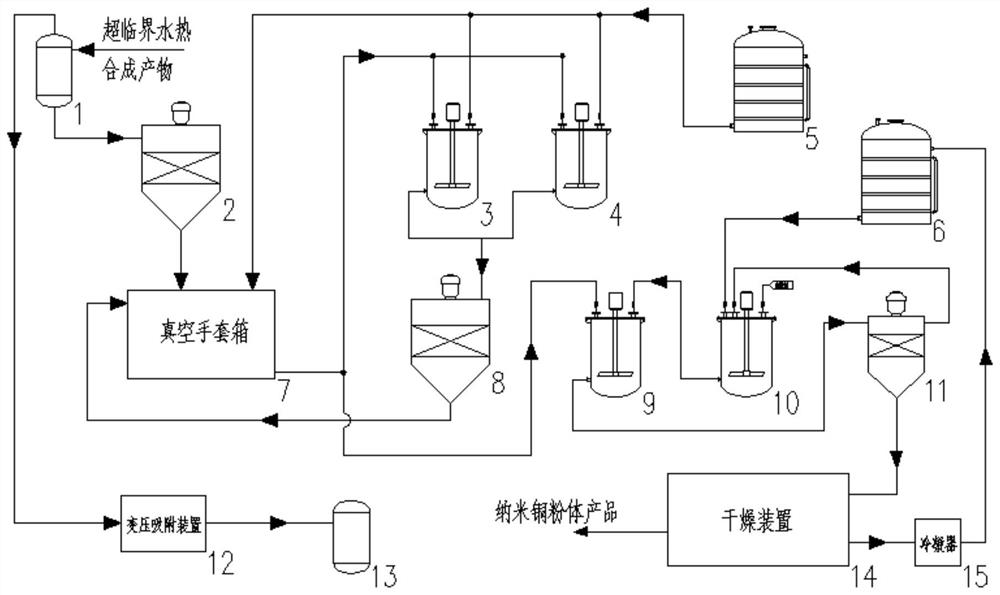
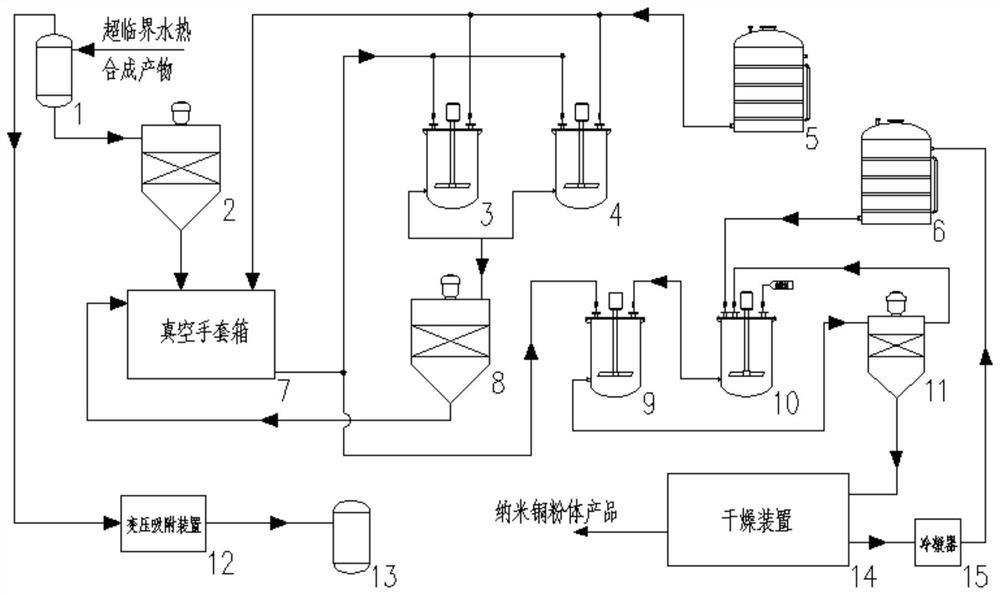
Continuous aftertreatment system and method for preparing nano materials through supercritical water thermal synthesis
ActiveCN111774580AReduce in quantityImprove running stabilityTransportation and packagingMetal-working apparatusContinuous flow centrifugationPhysical chemistry
The invention discloses a continuous aftertreatment system and method for preparing nano materials through supercritical water thermal synthesis. Through buffering cleaning tanks arranged in parallel,simultaneous cleaning of separated products in different batches and continuous running of a continuous flow centrifugal separator can be achieved, the production efficiency is effectively improved,and the number of facilities is reduced. Through the arrangement of a recycling system of a wrapping agent and a solvent of the wrapping agent, complete recycling of the wrapping agent and the wrapping agent solvent can be achieved, and the running economical efficiency of the system is obviously improved. Through the arrangement of a three-phase separator and a gas pressure changing adsorption device, gas-phase products in reaction products can be separated out, valuable gas in the gas-phase products is adsorbed and collected, and high economic benefits are brought.
Owner:XI AN JIAOTONG UNIV
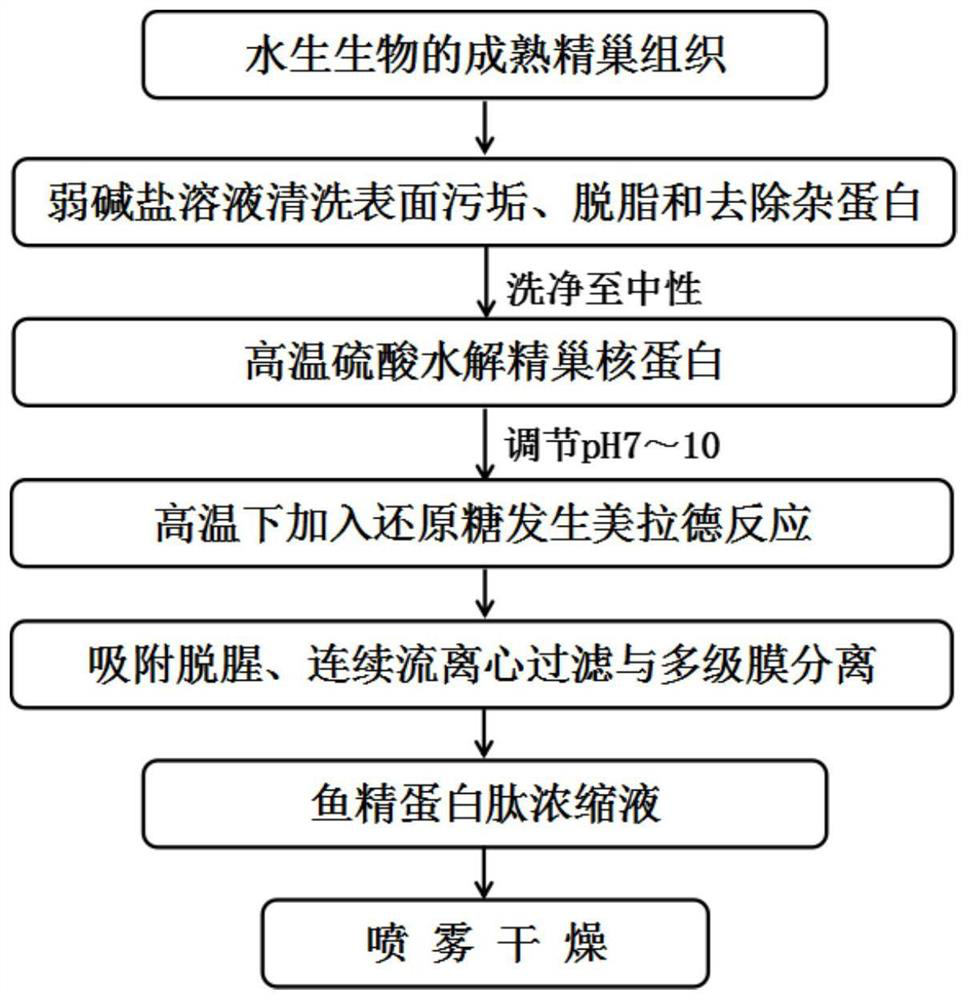
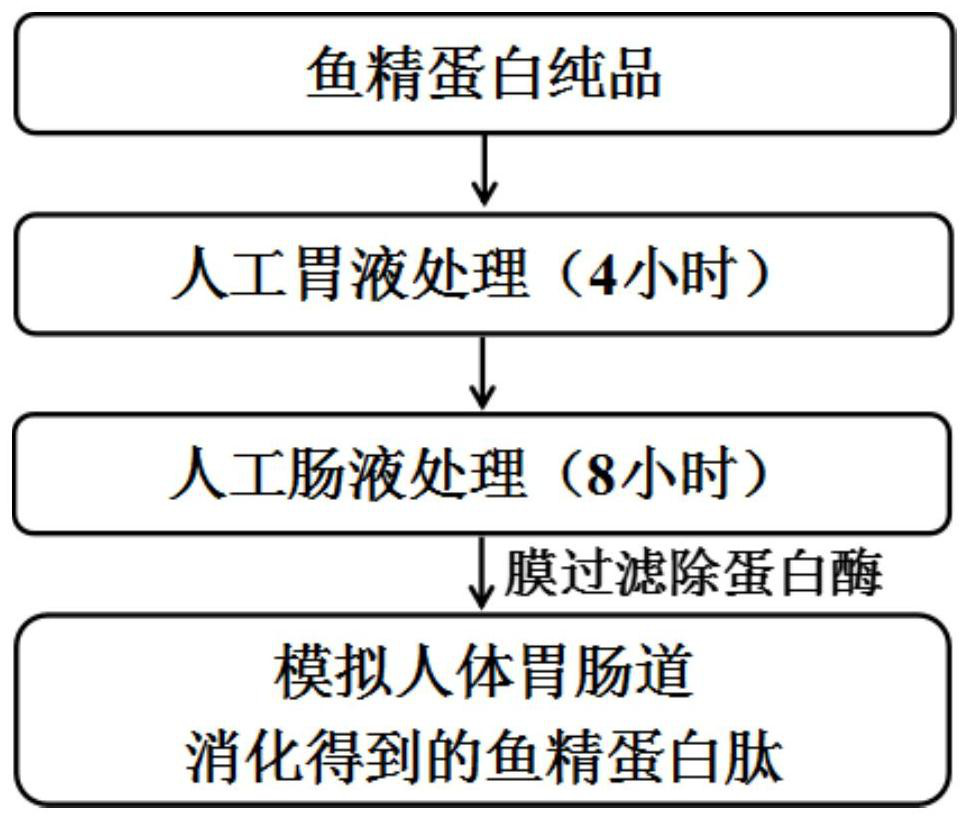
Large-scale preparation method of protamine peptide
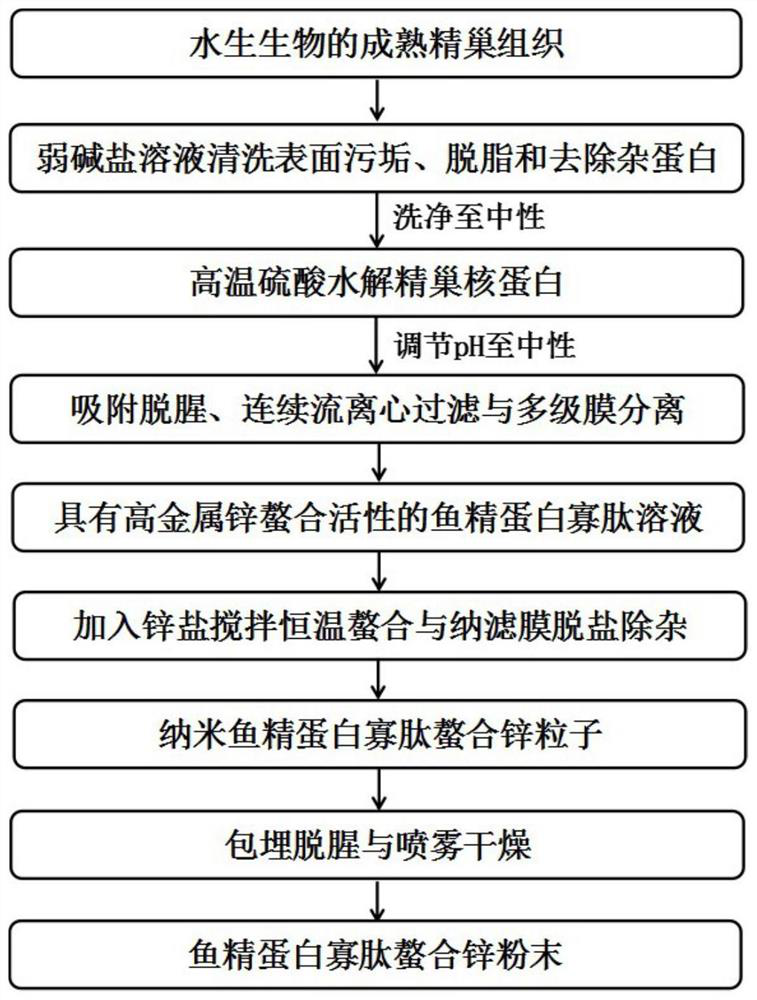
PendingCN113925110AHigh yieldReduce time consumptionAnimal proteins working-upChemical industryBiotechnologyMaillard reaction
The invention relates to a large-scale preparation method of protamine peptide. The method comprises the following steps: by taking mature testis tissues of aquatic organisms as raw materials, carrying out pretreatment by adopting a weak base salt solution for cleaning, then hydrolyzing testis nucleoprotein by adopting high-temperature sulfuric acid, adding reducing sugar under a high-temperature condition to carry out Maillard reaction, and then carrying out adsorption deodorization, continuous flow centrifugal filtration and multi-stage membrane separation to obtain a nucleotide-free protamine peptide concentrated solution with the content larger than or equal to 95%. Although a chemical extraction method is adopted, compared with the protamine peptide prepared by a current hot enzymolysis process, the protamine peptide prepared by the method disclosed by the invention is higher in yield and less in time consumption. Meanwhile, the production cycle of large-scale preparation of the protamine peptide can be basically controlled to be completed within 24 hours, so that the production cycle is shorter and more compact, the whole process is simpler and more efficient, and is more suitable for large-scale production. Compared with a protamine crude extract, the protamine peptide produced by the invention has better flavor in the aspects of smell and taste, and is easier to accept and eat by vast consumers.
Owner:THIRD INST OF OCEANOGRAPHY MINIST OF NATURAL RESOURCES
Production and purification process of a hemorrhagic fever vaccine
ActiveCN113337475BStable growthPromote growthSsRNA viruses negative-senseViral antigen ingredientsContinuous flow centrifugationVaccine Production
The invention provides a production and purification process of a hemorrhagic fever vaccine, which relates to the technical field of vaccine production. The production and purification process of the hemorrhagic fever vaccine comprises the following steps: cell passage, virus infection, virus liquid acquisition, virus liquid inactivation, continuous flow centrifugation , concentration by ultrafiltration, column chromatography and filtration. The process is simple to operate, the production process is stable, the side reaction is small, and the purified product has high purity.
Owner:罗益(无锡)生物制药有限公司
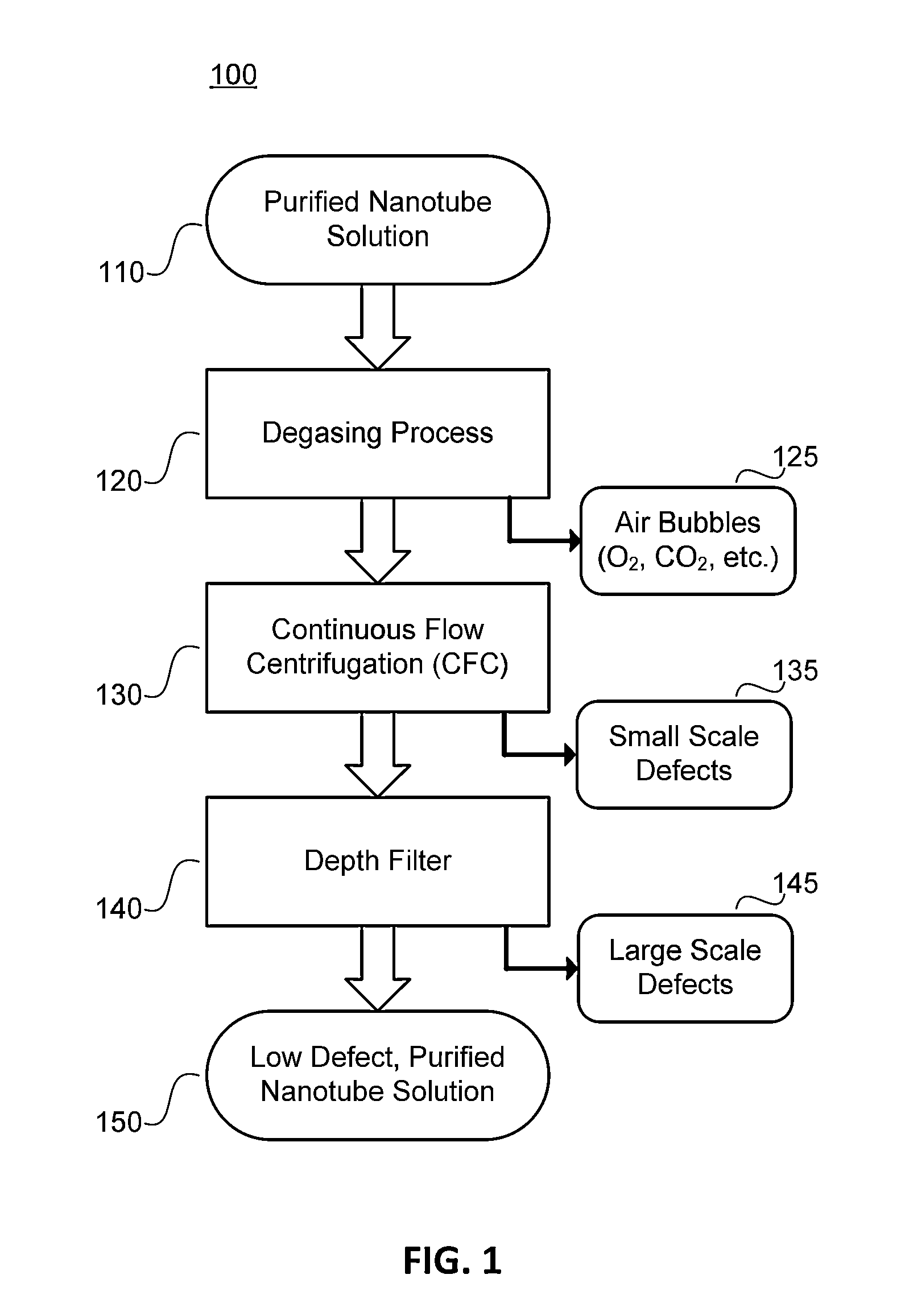
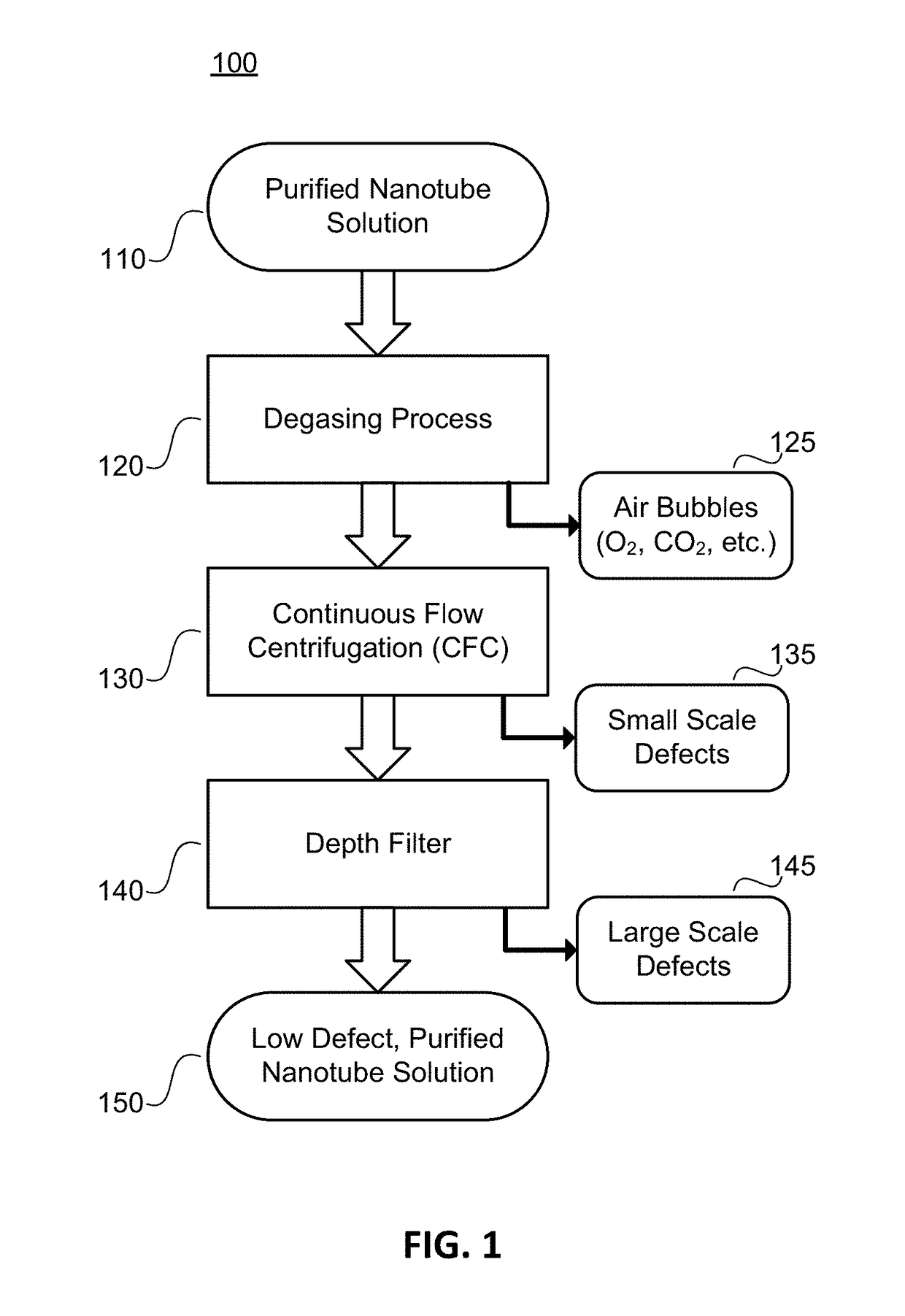
Low Defect Nanotube Application Solutions and Fabrics and Methods for Making Same
ActiveUS20140329430A1Low level of defectAvoid high levels of impuritiesNanotechLiquid degasification with auxillary substancesContinuous flow centrifugationEngineering
The present disclosure provides methods for removing defects nanotube application solutions and providing low defect, highly uniform nanotube fabrics. In one aspect, a degassing process is performed on a suspension of nanotubes to remove air bubbles present in the solution. In another aspect, a continuous flow centrifugation (CFC) process is used to remove small scale defects from the solution. In another aspect, a depth filter is used to remove large scale defects from the solution. According to the present disclosure, these three methods can be used alone or combined to realize a low defect nanotube application solutions and fabrics.
Owner:ZEON CORP +1
Preparation method of rotavirus inactivated vaccine
ActiveCN114717202AAvoid high concentration processPromote degradationViral antigen ingredientsArtificial cell constructsContinuous flow centrifugationVirus inactivation
The invention relates to a preparation method of a rotavirus inactivated vaccine, and belongs to the technical field of biological medicines. The invention provides a preparation method of a rotavirus inactivated vaccine, the preparation method comprises the steps of virus release, virus clarification, virus inactivation, host DNA degradation, virus concentration and virus purification, the virus release step adopts a pressure crushing method to crush host cells, the virus clarification step adopts continuous flow centrifugation, and the virus purification step adopts continuous flow centrifugation to purify the host cells. The virus purification step adopts continuous flow sucrose density gradient centrifugation; according to the preparation method, a pressure crushing method is adopted to crush host cells in the virus release step, compared with freeze thawing crushing, the pressure crushing step is simple, continuous sample injection can be achieved, the operation time is greatly shortened, compared with ultrasonic crushing, the virus activity is not prone to being damaged through pressure crushing, and the crushing effect on the host cells is better; in addition, closed-loop sterile operation can be achieved through pressure crushing, the crushing condition is stable, and large-scale industrial production can be better achieved.
Owner:BEIJING CELL FUSION BIOTECHNOLOGY CO LTD
A method for large-scale production of porcine pseudorabies inactivated vaccine
ActiveCN107267466BHigh purityLow costViral antigen ingredientsInactivation/attenuationMolecular sieveAdjuvant
The invention belongs to the technical field of vaccines and relates to a method for large-scale production of a porcine pseudorabies inactivated vaccine. The method comprises preparing a virus solution of porcine pseudorabies (XF-1 strain), carrying out continuous flow centrifugation, hollow fiber column clarification filtration, hollow fiber column ultrafiltration concentration and Sepharose 4FF molecular sieve gel chromatography purification treatment to obtain purified porcine pseudorabies viruses, adding a formaldehyde solution having a final concentration of 0.4% (v / v) into the purified porcine pseudorabies viruses, carrying out inactivation at 37 DEG C for 48h, and carrying out emulsification with a 201 adjuvant to obtain the porcine pseudorabies inactivated vaccine. The porcine pseudorabies inactivated vaccine can well prevent highly pathogenic mutant pseudorabies prevailing in the market.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
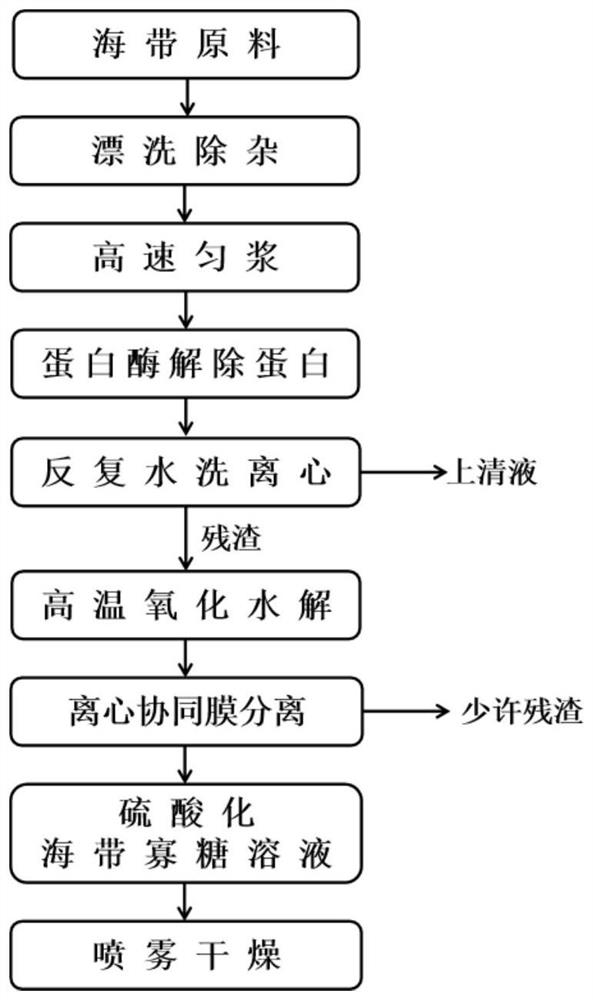
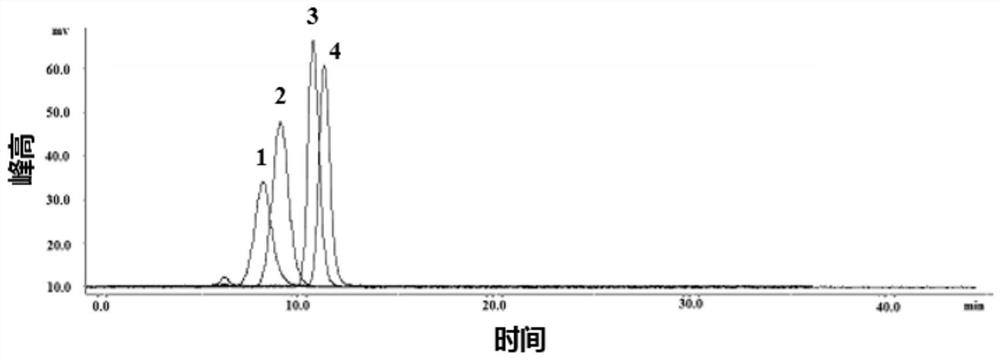
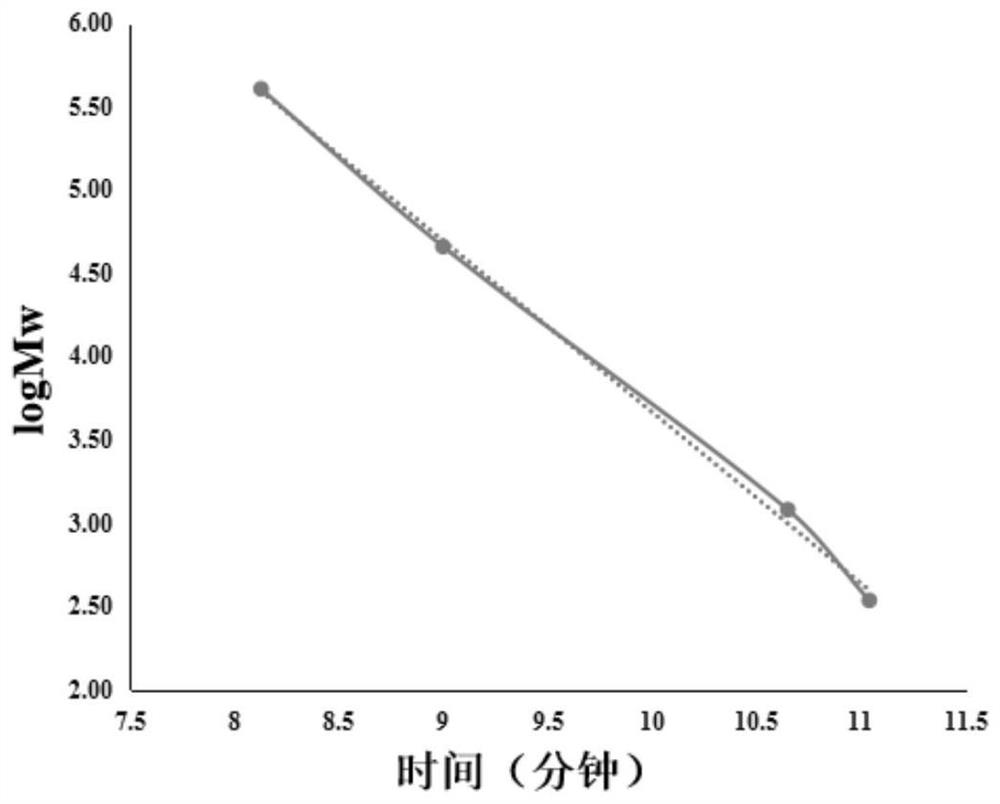
Large-scale preparation method of sulfated kelp oligosaccharide suitable for cosmetics
ActiveCN112521430AQuick removalSolve pollutionCosmetic preparationsSugar derivativesSulfationContinuous flow centrifugation
The invention relates to a large-scale preparation method of sulfated kelp oligosaccharide suitable for cosmetics. The method comprises the following steps of: by taking kelp as a raw material, performing pretreatment, namely quickly rinsing and degreasing by adopting an alkali solution, performing high-speed homogenate grinding and protease enzymolysis to quickly remove all crude proteins and partial mannitol and ash, performing high-temperature oxidative hydrolysis, quickly preparing the pretreated kelp residues into a sulfated kelp oligosaccharide primary extract, separating and purifying the sulfated kelp oligosaccharide extract at normal temperature by utilizing a continuous flow centrifugation and membrane separation technology, thereby obtaining a sulfated kelp oligosaccharide solution, and finally, quickly drying by utilizing a spray drying technology, thereby forming the sulfated kelp oligosaccharide powder. The method disclosed by the invention is simple and feasible in overall process, high in efficiency, energy-saving and suitable for large-scale production; the prepared sulfated kelp oligosaccharide is high in molecular weight concentration ratio and even can be comparable with a dextran standard product; and moreover, the prepared sulfated kelp oligosaccharide with the molecular weight of less than 1000 is particularly suitable for being used in various cosmetics.
Owner:THIRD INST OF OCEANOGRAPHY MINIST OF NATURAL RESOURCES
Patch for promoting wound healing and preparation method thereof
PendingCN113876810AEasy to prepareLow instrument requirementsCell dissociation methodsSkeletal/connective tissue cellsWound healingContinuous flow centrifugation
The invention discloses a preparation method of a patch for promoting wound healing. According to the invention, an ultralow temperature cell lysis technology is combined with continuous flow centrifugation to separate out active components having a promoting effect on wound healing in MSCs, the active components for promoting wound healing are finally obtained by optimizing process parameters of the active components for wound healing, the active components are bound on a carrier patch for freeze-drying, the patch containing stem cell active ingredients and capable of promoting wound healing can be provided, the preparation method is simple, the requirement for instruments is not high, the patch is suitable for mass production and convenient to use, umbilical cord mesenchymal stem cells serve as the active ingredients of the patch, the use allergy rate is low, and the application range is wide.
Owner:陕西医赛尔生物科技有限公司
A continuous post-processing system and method for preparing nanomaterials by supercritical hydrothermal synthesis
ActiveCN111774580BReduce in quantityImprove running stabilityTransportation and packagingMetal-working apparatusContinuous flow centrifugationPhysical chemistry
The invention discloses a continuous post-processing system and method for preparing nanomaterials by supercritical hydrothermal synthesis. By setting buffer cleaning tanks in parallel, simultaneous cleaning of different batches of separated products and continuous operation of continuous flow centrifuges can be realized. Effectively improve production efficiency and reduce the number of equipment. By setting up a recovery system for the coating agent and its solvent, the complete recycling of the coating agent and the solvent of the coating agent can be realized, and the economical efficiency of the system operation can be significantly improved. By setting up a three-phase separator and a gas pressure swing adsorption device, the gas phase product in the reaction product can be separated, and the valuable gas can be collected by adsorption, which brings great economic benefits.
Owner:XI AN JIAOTONG UNIV
Nanotube application deposition system for forming low defect nanotube fabrics
InactiveUS20170246561A1NanotechLiquid degasification with auxillary substancesContinuous flow centrifugationEngineering
Owner:ZEON CORP
Continuous flow centrifugal device
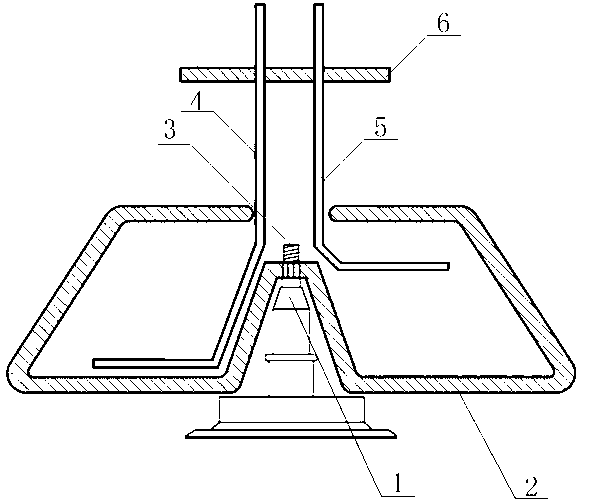
PendingCN114210469AReduce wear rateLow pollution rateCentrifugesContinuous flow centrifugationEngineering
The invention relates to a continuous flow centrifugal device, and relates to the technical field of centrifugal devices, the continuous flow centrifugal device comprises a machine body and a rotating bowl, a driving device for driving the rotating bowl to rotate is arranged on the machine body, a sealing device is arranged on the machine body, and the sealing device comprises an annular box arranged on the rotating bowl; the fixed cover is arranged on the machine body and extends into the annular box, a sealing cavity is formed between the fixed cover and the inner side wall of the annular box, and a liquid inlet pipe and a liquid outlet pipe which are used for liquid inlet and liquid outlet respectively are arranged on the fixed cover; the liquid supply mechanism is arranged on the machine body and used for adding liquid into the sealing cavity to seal the rotating bowl. The rotating bowl and the fixed cover are physically separated through the liquid, so that the probability of abrasion caused by rotation of the rotating bowl and the sealing piece is reduced under the condition that the sealing function is achieved, the probability that the stock solution is polluted by chippings is reduced, and the safety of the stock solution is improved.
Owner:上海泰闪科技有限公司
A method for preparing live attenuated mumps vaccine
ActiveCN113999825BReduce quality and safety risksGood removal effectSsRNA viruses negative-senseCell dissociation methodsContinuous flow centrifugationAttenuated vaccine
The invention relates to a method for preparing live attenuated mumps vaccine, which belongs to the technical field of biomedicine. The invention provides a method for preparing a live attenuated mumps vaccine, the method comprising a clarification step using continuous flow centrifugation under the conditions of a fixed centrifugal force of 4000-14000g and a sample loading speed of 400-1500mL / min , to clarify the virus harvest liquid of the attenuated mumps virus strain; compared with the clarification methods (membrane filtration and silk cloth filtration) of the existing mumps live attenuated vaccine, the clarification effect of the method is more stable and the degree of automation is higher Higher, easier to operate, easier to achieve aseptic operation, which helps to reduce the quality and safety risks of live attenuated mumps vaccines, and the method has the best removal effect on residual cell debris in the virus harvesting liquid, and has the best effect on the virus The impact of infectious mumps virus titer in the harvest fluid is minimal, which is helpful to obtain live attenuated mumps vaccine with high virus titer.
Owner:BEIJING CELL FUSION BIOTECHNOLOGY CO LTD +1
A method for large-scale production of high-purity porcine pseudorabies virus
The invention belongs to the technical field of vaccines and in particular relates to a method for large-scale production of a high-purity porcine pseudorabies virus. The method comprises processes of continuous flow centrifugation, hollow fiber clarification filtering, ultrafiltration and concentration, and molecular sieve purification. The virus recycling rate is increased to the maximum extent, and the content of impurity proteins is reduced. A porcine pseudorabies virus concentrated solution and purified antigen produced by using the method are particularly applicable to vaccine preparation, and compared with a porcine pseudorabies virus inactivated vaccine produced according to the prior art, the high-purity porcine pseudorabies virus is relatively high in safety, relatively high in uniformity and relatively good in immune effect, and side reactions of vaccines are fundamentally reduced.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Large-scale preparation method of protamine oligopeptide chelated zinc
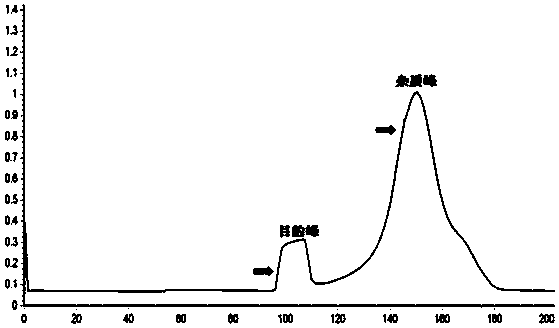
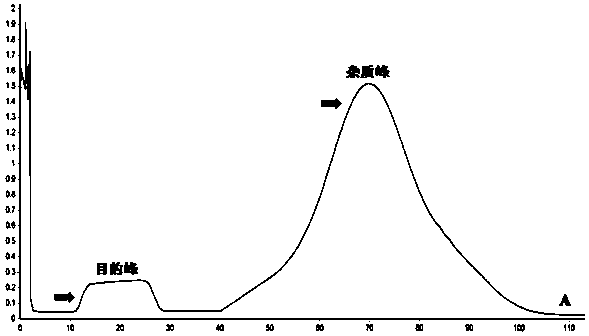
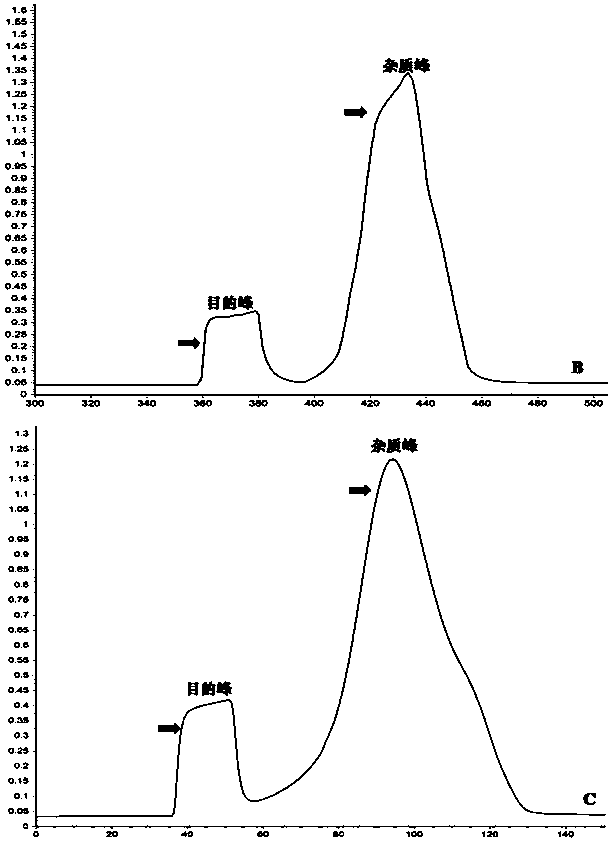
ActiveCN114478738AHigh yieldReduce time consumptionGeneral water supply conservationAccessory food factorsContinuous flow centrifugationChelated zinc
The invention relates to a large-scale preparation method of protamine oligopeptide chelated zinc. The method comprises the following steps: by taking mature testis tissues of aquatic organisms as raw materials, hydrolyzing testis nucleoprotein by adopting high-temperature sulfuric acid, carrying out continuous flow centrifugal filtration and multi-stage membrane separation to obtain a protamine oligopeptide solution which has the content of more than or equal to 95%, does not have nucleotide and has high metal zinc chelating activity, then adding zinc salt, stirring, carrying out constant-temperature chelating, and carrying out nanofiltration membrane desalination and impurity removal, thereby obtaining the protamine oligopeptide with high metal zinc chelating activity. The particle size of the nano protamine oligopeptide chelated zinc particles is less than 300nm, and most (more than 80%) molecular weights of the nano protamine oligopeptide chelated zinc particles are less than 1000Da. Although a chemical extraction and metal chelation method is adopted, compared with a commonly used enzymolysis extraction and metal chelation method, the protamine oligopeptide chelated zinc obtained by the method disclosed by the invention is higher in yield, less in time consumption and higher in chelation rate. Meanwhile, the production of large-scale preparation of the protamine oligopeptide chelated zinc does not need special instruments and equipment, the technological method is simple and reliable, the technological process is efficient and flexible, and the protamine oligopeptide chelated zinc subjected to adsorption deodorization and embedding deodorization is good in flavor in the aspects of smell and taste, is easier to serve as a food additive or a nutritional supplement, and is suitable for industrial production. The product is accepted and eaten by vast consumers; and the protamine oligopeptide chelated zinc which is not subjected to fishy smell removal treatment can still be used as a feed additive.
Owner:THIRD INST OF OCEANOGRAPHY MINIST OF NATURAL RESOURCES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com