A kind of preparation method of foot-and-mouth disease purified vaccine
A culture medium and cell technology, applied in the biological field, can solve the problems of large immune side effects, short immune period, and low antibody level, and achieve the effect of eliminating emergency response, ensuring safety, and ensuring safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The production of embodiment 1 foot-and-mouth disease virus liquid
[0029] 1. Culture of BHK-21 cells
[0030] Prepare serum-free or animal-derived component-free medium (1 L) according to the following recipe:
[0031] 380mgL-Alanine, 90mgL-Arginine Hydrochloride, 280mgL-Aspartic Acid, 70mgL-Cysteine Hydrochloride, 120mgL-Glutamic Acid, 50mgL-Arginine, 100mgL-Tyrosine, 130mgL-Valine Acid, 2.5g sucrose, 100mg sodium citrate, 7.5gNaCl, 300mgKCl, 200mgCaCl 2 And 20mg of phenol red, distilled water to make up 1000ml.
[0032] Serum-free Microcarrier Suspension Culture of BHK-21 Cells BHK-21 cells are from ATCC, the working cell bank cell passage should not exceed 10 passages of the main cell bank, and the BHK-21 working cells should be within 10 passages. Serum-free culture medium of the present invention is added Cytodex-3 microcarrier according to 5~8g / ml, cultivates 6-7 days with 1.5L bioreactor 37 ℃, stirring speed 20rpm, carries out digestion again, transfers to...
Embodiment 2
[0043] The inactivation of embodiment 2 purifying foot-and-mouth disease virus
[0044] The purified virus solution and the crude virus solution prepared in Example 1 were sterilized and filtered with a 0.22 μm membrane and added with 1 / 4000 β propiolactone to inactivate at 37°C for 3-4 days, and stored at 4°C for use.
Embodiment 3
[0045] Example 3 Vaccine Preparation
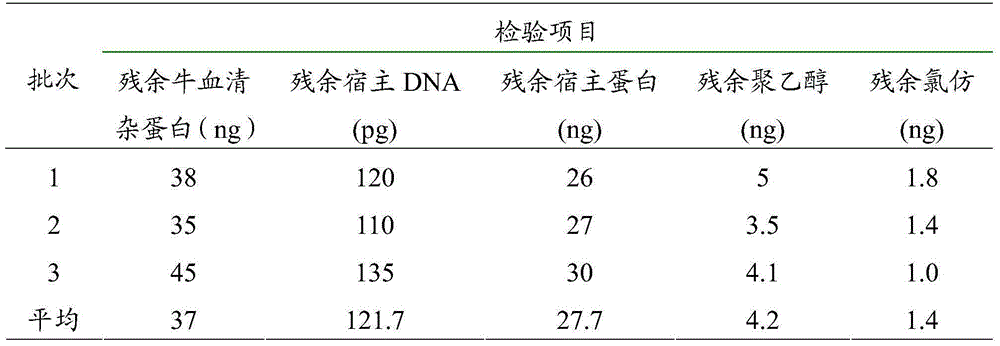
[0046] The inactivated purified inactivated solution and crude virus solution were diluted with 199 medium (pH 7.2) and subpackaged to contain 20 μg of each serotype antigen per head. See Table 2 for the comparison between the prepared purified vaccine and the crude vaccine.
[0047] Table 2 Purified foot-and-mouth disease vaccine-induced pig immune response
[0048]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com