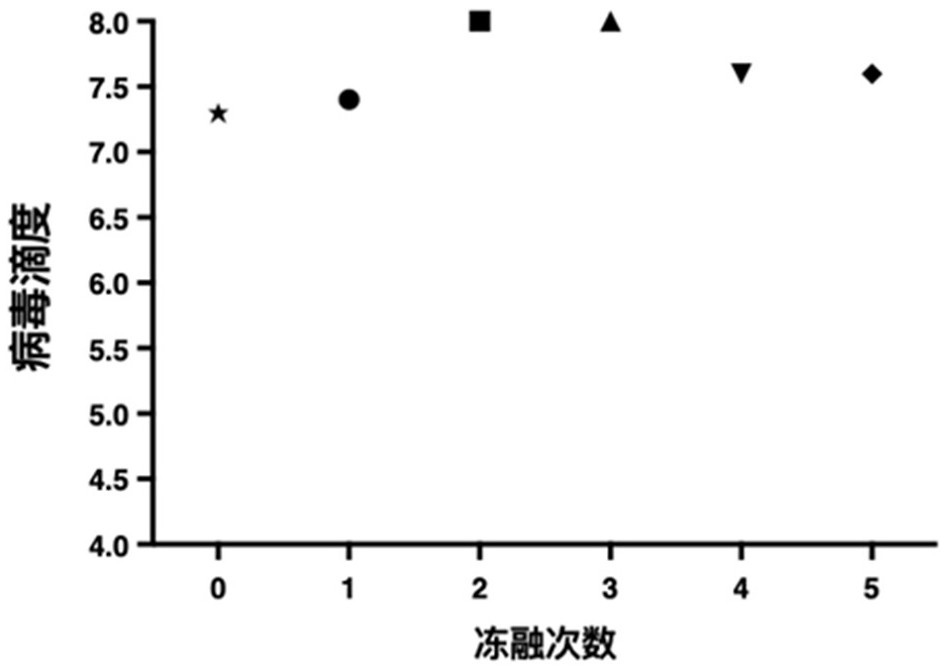
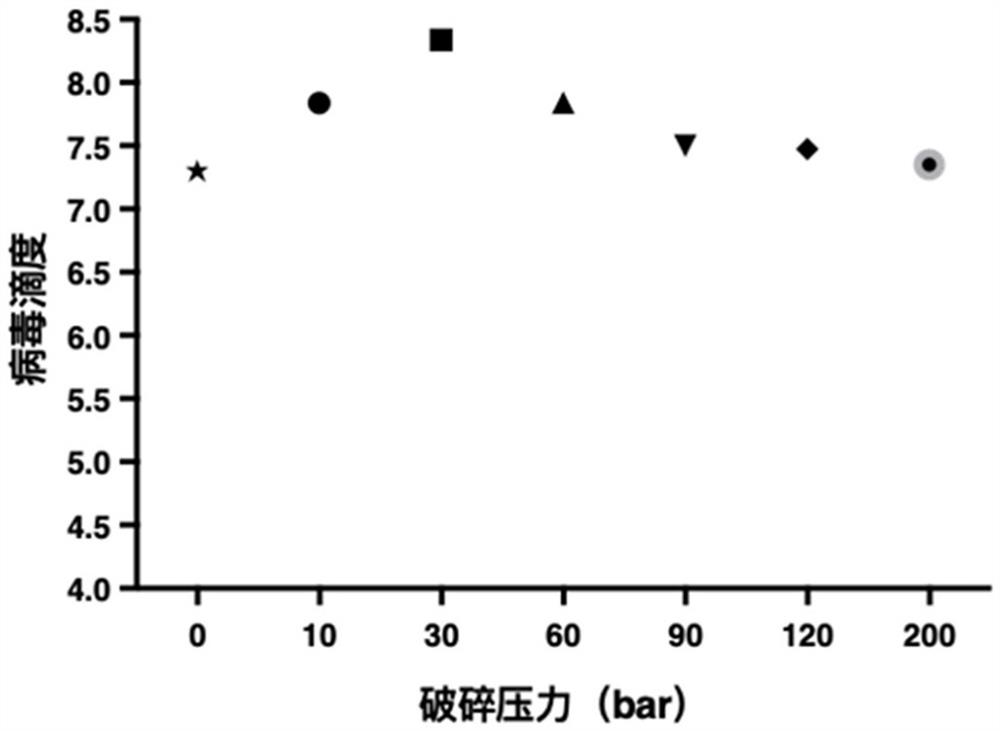
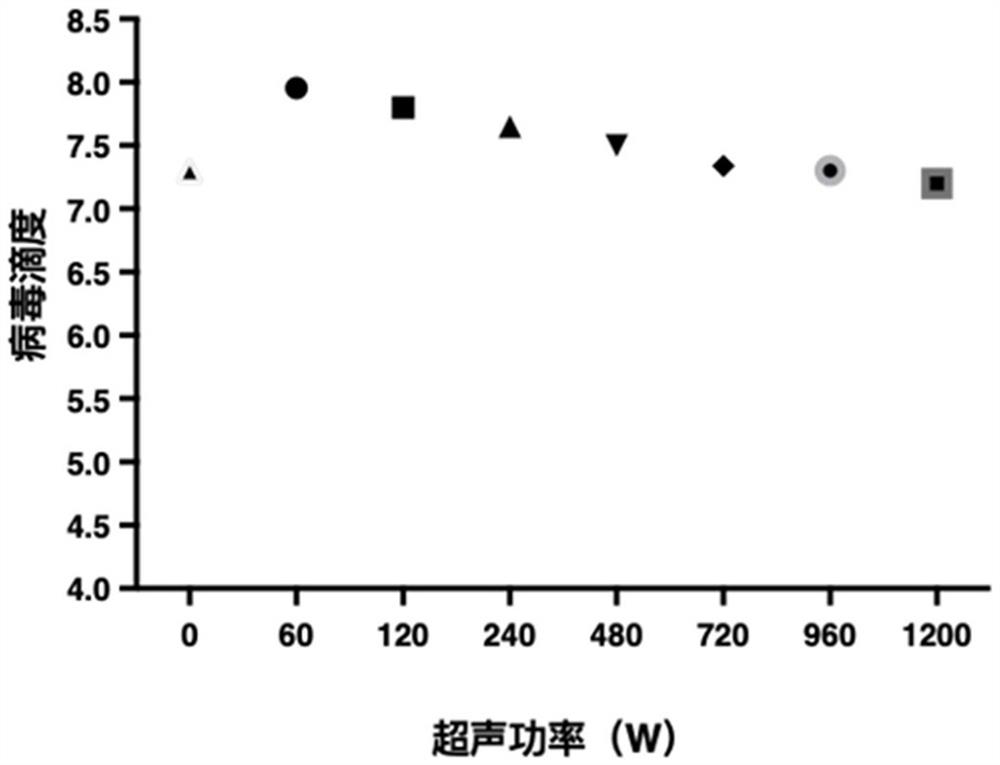
Preparation method of rotavirus inactivated vaccine
A rotavirus and virus inactivation technology, applied in the field of biomedicine, can solve the problems of easy-to-break virus activity, high heat, and long operation cycle, and achieve the effects of reducing virus structure damage, shortening operation time, and saving concentration time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0086] The preparation method of the rotavirus virus culture liquid involved in the following examples comprises the following steps:
[0087] Host cell preparation: Vero cells (purchased from ATCC) were prepared with cell growth medium to a cell density of 1.8 × 10 6 After cells / mL of cell suspension, 1.5L of cell suspension was added to a 10L bioreactor (purchased from Hectopascal) containing 8.5L of cell growth medium and 40g of microcarriers (Cytodex-1, purchased from GE). The cells were cultured for 24 h under the conditions of temperature 37 °C, pH 7.2, dissolved oxygen 50%, and stirring speed 45 rpm; after the incubation, the Vero cells in the bioreactor were perfused with cell growth solution, so that the glucose in the cell growth solution was perfused. The content is not less than 2g / L, continuous perfusion for 120h, and a total of 10L of cell growth solution is perfused until Vero cells are covered with microcarriers; the cell growth solution is composed of DMEM med...
Embodiment 1
[0093] Embodiment 1: a kind of preparation method of rotavirus inactivated vaccine
[0094] The present embodiment provides a preparation method of a rotavirus inactivated vaccine, and the preparation method comprises the following steps:
[0095] Carrier separation: filter the virus culture liquid in the bioreactor with a filter screen with a pore size of 106 μm (purchased from Hectopas) to remove the microcarriers to obtain a virus separation liquid;
[0096] Virus release: Use a pressure crusher (purchased from Yonglian Biotechnology (Shanghai) Co., Ltd., model UR-96) to crush the host cells in the virus separation solution at a crushing pressure of 10 bar and an influent flow rate of 100 mL / min. Release the rotavirus from the host cell to obtain a virus harvest solution;
[0097] Virus clarification: Using a high-speed refrigerated centrifuge (purchased from Hitachi, model CR22N), the virus harvest solution was subjected to continuous flow centrifugation at a fixed centri...
Embodiment 2
[0102] Embodiment 2: a kind of preparation method of rotavirus inactivated vaccine
[0103] The present embodiment provides a preparation method of a rotavirus inactivated vaccine, and the preparation method is:
[0104] On the basis of Example 1, the crushing pressure was replaced with 20 bar.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com