Large-scale preparation method of recombinant staphylococcus aureus vaccine
A staphylococcus, golden yellow technology, applied in the field of protein stock solution, can solve the problems of rapid spread of drug resistance, difficult to control, high incidence of nosocomial infection of Staphylococcus aureus, and incapable of large-scale preparation, etc., and achieves a simple preparation method and high quality Good, realize the effect of industrialized scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
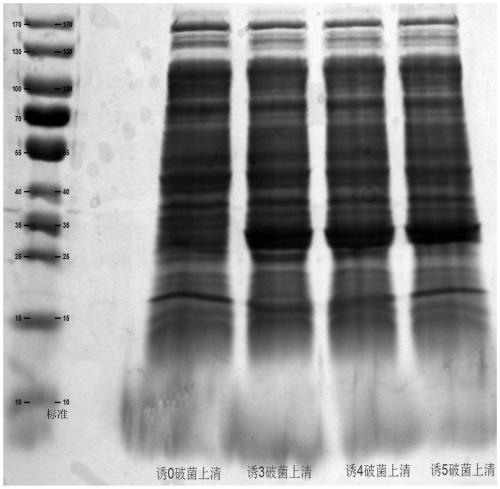
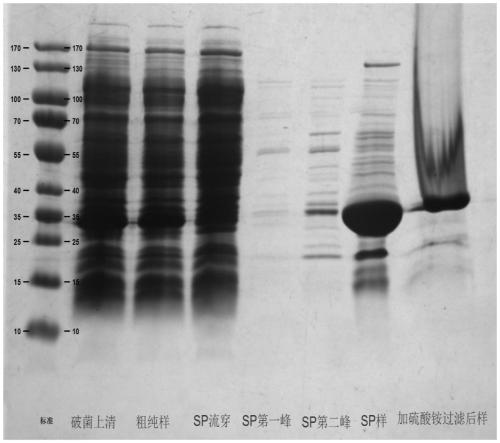
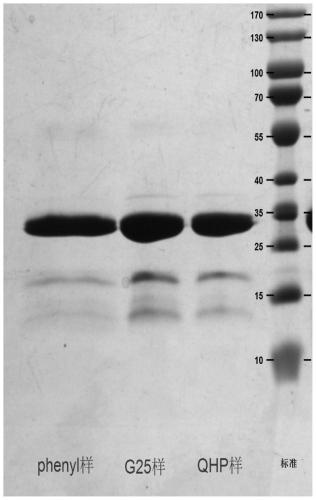
[0044] The large-scale preparation method of recombinant Staphylococcus aureus vaccine of the present invention comprises the following steps:
[0045] (1) mSEB protein working seed batch strains were opened and inoculated in a Erlenmeyer flask. The inoculum size is strain: culture medium = 1:100 (ml:ml), and each Erlenmeyer flask is filled with 25±2ml LB liquid medium. Place it in a constant temperature shaking incubator at 36-38°C, and cultivate it at 180-300rpm for 6-10 hours, and it is the first-generation production strain.
[0046] (2) The first-generation production strain is inoculated in the seed tank for second-generation culture. The seed tank is filled with 30L of LB liquid medium, the inoculation amount is the first-generation strain: culture medium = 1:2000 (ml:ml), the parameters of the control seed tank are temperature: 36~38°C, 50~500rpm, air flow: 30~ 70L / min, pH: 7.2-7.5 and cultured for 6-10 hours, it is the second-generation production strain. During th...
Embodiment 2
[0063] (1) HI protein working seed batch strains were opened and inoculated in a Erlenmeyer flask. The inoculum size is strain: culture medium = 1:100 (ml:ml), and each Erlenmeyer flask is filled with 25±2ml LB liquid medium. Place it in a constant temperature shaking incubator at 36-38°C, and cultivate it at 180-300rpm for 6-10 hours, and it is the first-generation production strain.
[0064](2) The first-generation production strain is inoculated in the seed tank for second-generation culture. The seed tank is filled with 30L of LB liquid medium, the inoculation amount is the first-generation strain: culture medium = 1:2000 (ml:ml), the parameters of the control seed tank are temperature: 36~38°C, 50~500rpm, air flow: 30~ 70L / min, pH: 7.2-7.5 and cultured for 6-10 hours, it is the second-generation production strain. During the cultivation process, samples were taken every hour to detect the OD600 value. When the OD600 value was greater than 1.5, the seed tank cultivation ...
Embodiment 3
[0080] (1) MntC protein working seed batch strains were opened and inoculated in a Erlenmeyer flask. The inoculum size is strain: culture medium = 1:100 (ml:ml), and each Erlenmeyer flask is filled with 25±2ml LB liquid medium. Place it in a constant temperature shaking incubator at 36-38°C, and cultivate it at 180-300rpm for 6-10 hours, and it is the first-generation production strain.
[0081] (2) The first-generation production strain is inoculated in the seed tank for second-generation culture. The seed tank is filled with 30L of LB liquid medium, the inoculation amount is the first-generation strain: culture medium = 1:2000 (ml:ml), the parameters of the control seed tank are temperature: 36~38°C, 50~500rpm, air flow: 30~ 70L / min, pH: 7.2-7.5 and cultured for 6-10 hours, it is the second-generation production strain. During the cultivation process, samples were taken every hour to detect the OD600 value. When the OD600 value was greater than 1.5, the seed tank cultivati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com