Method for large-scale production of porcine pseudorabies inactivated vaccine
A technology for porcine pseudorabies and porcine pseudorabies virus, which is applied in the field of vaccines, can solve the problems of increased residual concentration of harmful substances, large side reactions in vaccine products, and increased effective antigen content, so as to reduce the degree and probability of side reactions, and improve social Benefit and economic benefits, the effect of reducing the number of production times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1 large-scale production prepares porcine pseudorabies virus (XF-1 strain)
[0041] The concentration of 300L is 4.0~10.0×10 6 Each / ml BHK-21 cell suspension was inoculated in a 650L bioreactor, and 350L of BHK-21 medium containing 0.5% to 2% (v / v) newborn bovine serum was added, and the cell culture temperature was controlled at 36°C to 37°C. ℃, pH6.5~7.5, stirring speed 80rpm~150rpm, dissolved oxygen concentration 30%~60%, reactor parameter setting four-way gas, clean air, oxygen, nitrogen and CO 2 , according to the culture time, cell density and pH value, set different gas parameter ranges. After culturing for 24 hours, the cell count and viability were observed by trypan blue staining. After 2 days of suspension culture, new BHK-21 medium should be supplemented according to 5% (v / v) of the total culture volume in the bioreactor. The cells were cultured for 3 days, and the density reached 4.0~10.0×10 6 cells / ml to obtain BHK-21 cell suspension.
[00...
Embodiment 2
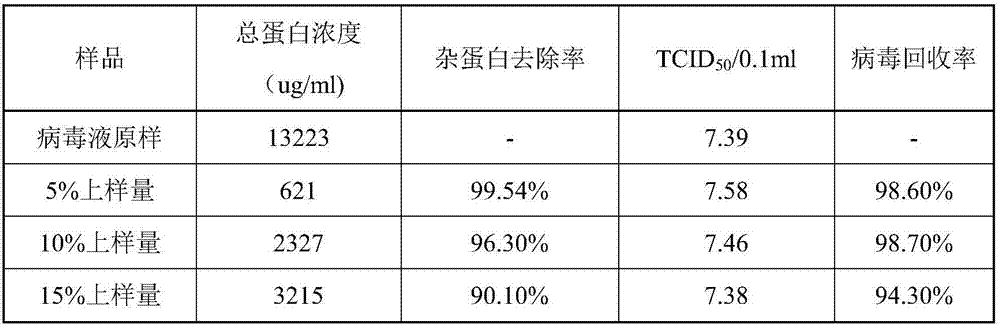
[0043] The purification of embodiment 2 porcine pseudorabies virus liquid
[0044] 1. Hollow fiber column clarification process
[0045] 1 system pretreatment
[0046] 1.1 Install the 0.45 μm hollow fiber column into the hollow fiber column control equipment, connect the corresponding pipeline, and infiltrate the hollow fiber column with sterile injection water for 30 minutes after assembly.
[0047] 1.2 System Integrity Detection
[0048] The pressure hold method checks the integrity of the system.
[0049] 1.3 System processing
[0050] Cleaning and sterilization: Use sterile 0.5mol / L NaOH solution to sterilize the system for 30 minutes, then clean the system with sterile water for injection to remove residual alkali solution until the pH is 7.0;
[0051] 1.4 Detection of water flux
[0052] Use sterile water for injection to pass through at the specified pump speed, and calculate the water flux of the hollow fiber column at the corresponding temperature.
[0053] 1.5 ...
Embodiment 3
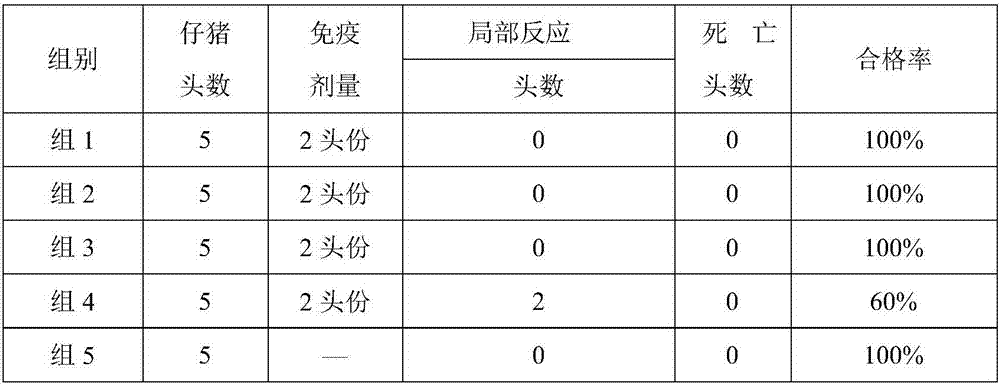
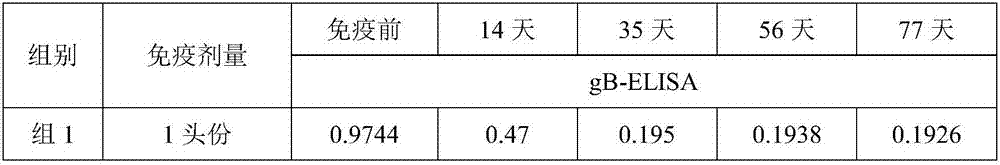
[0081] The preparation of embodiment 3 porcine pseudorabies inactivated vaccine
[0082] After purification of porcine pseudorabies virus (XF-1 strain), the antigenic virulence is at 10 8.0 TCID 50 / ml or more, add formaldehyde solution with a final concentration of 0.4% (v / v) to inactivate at 37°C for 48 hours, and store it at 4°C for use after passing the relevant inspection according to the appendix of the current "Chinese Veterinary Pharmacopoeia".
[0083] After the qualified porcine pseudorabies virus (XF-1 strain) is diluted according to the requirements of the vaccine, it is mixed with MONTANIDE respectively TM ISA 201 adjuvant was emulsified according to the mass ratio of 1:1 (g / g), the stirring speed was 500rpm / min, and the emulsification time was 30min. The antigen content in the final porcine pseudorabies inactivated vaccine is 10 6.0 TCID 50 / head portion, 10 7.0 TCID 50 / head portion and 10 7.5 TCID 50 / first portion, after the emulsification is complet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com