Method for reducing cell protein and DNA (Deoxyribose Nucleic Acid) residues in rabies virus product
A rabies virus and cell protein technology, applied in the field of reducing cell protein and DNA residues in rabies virus products, can solve problems such as nuclease residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1. Preparation of human rabies vaccine with Vero cells and the removal method of Vero cell protein and DNA
[0064] 1. Experimental materials and equipment
[0065] After culturing Vero cells using basket bioreactor and sheet carrier culture technology, inoculate virus culture to prepare virus liquid, and the materials used are shown in Table 1.
[0066] Table 1
[0067] name Material Vero cells Seeding density: 5×10E+6 / ml strain rabies virus PM strain cell culture medium Gibco Serum Free Medium (VP SFM AGT) virus maintenance medium Ordinary 199 Medium filter element 0.6μm micropore diameter
[0068] 2. Experimental instruments and equipment
[0069] The virus liquid is concentrated by ultrafiltration through a 300KD membrane to obtain a virus concentrate, inactivated and hydrolyzed, subjected to molecular sieve chromatography, and then added with a stabilizer to obtain a stock solution. The equipment used...
Embodiment 2
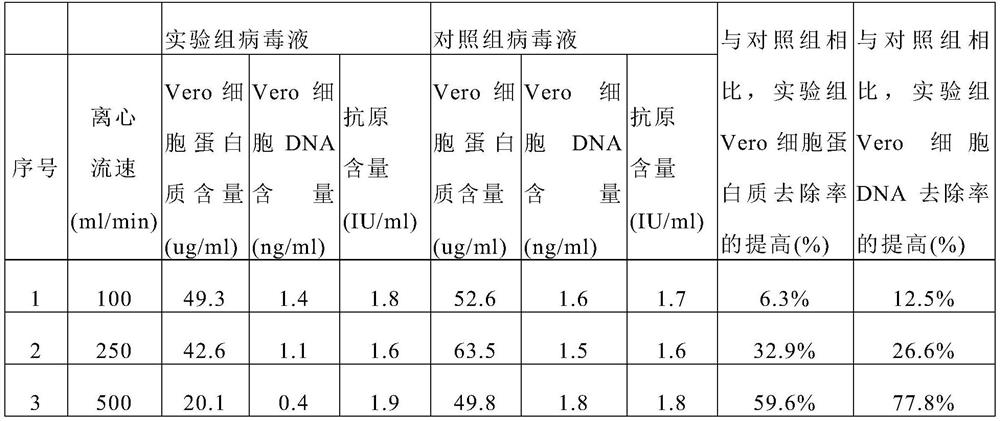
[0094] Example 2. Protein and DNA removal under various centrifugal flow rates (sample before ultrafiltration)
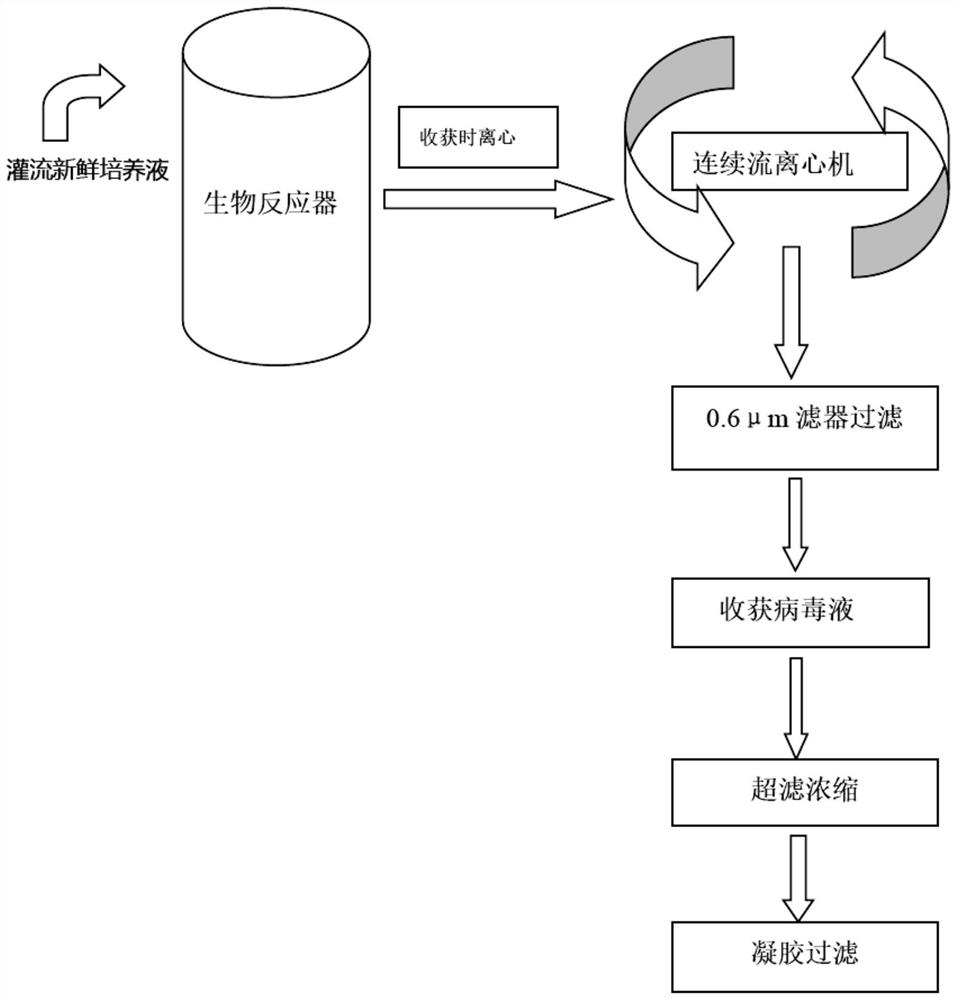
[0095] Experimental group: According to the technique of Example 1, after culturing for 48 hours in "(3) Circulating and centrifuging the viral supernatant in the tank", filter through a 0.6 μm microfiltration membrane, and then harvest the virus liquid for measurement ( figure 2 , before ultrafiltration concentration).
[0096] Control group: Cell culture and virus culture were carried out according to the process of Example 1, wherein "(3) Circulating centrifugation of virus supernatant in the tank" was not carried out, and the virus liquid was directly harvested and then subjected to continuous flow centrifugation and 0.6 μm microfiltration membrane filtration .
[0097] In the process of culturing the virus in the bioreactor, the centrifugal force was 10000g, the centrifugal flow rate was 100ml / min (No. 1), 250ml / min (No. 2), 500ml / min (No. 3), and then filter...
Embodiment 3
[0103] Embodiment 3, the mass analysis of sample after ultrafiltration concentration
[0104] Experimental group: The same operation was performed as the experimental group in Example 2 (the supernatant was centrifuged at a centrifugal flow rate of 500 ml / min), and ultrafiltration concentration and gel filtration were also performed after the virus was harvested.
[0105] Control group: The same operation was performed as the control group in Example 2 (the supernatant was centrifuged at a centrifugal flow rate of 500 ml / min), and ultrafiltration concentration and gel filtration were also performed after the virus was harvested.
[0106] The virus solutions of the experimental group and the control group were concentrated by 40 times of concentration and ultrafiltration with a 300KD membrane package, and then purified by a Sepharose 4FF gel chromatography column, and then measured.
[0107] Table 5 shows the contents of Vero cell protein, Vero cell DNA and antigen in the origi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com