Serum-free medium suitable for large-scale production of influenza vaccines
A serum-free medium and influenza vaccine technology, applied in the field of serum-free medium, can solve the problems of long cycle, inability to completely remove substances, unsuitable cell culture and production of biological products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
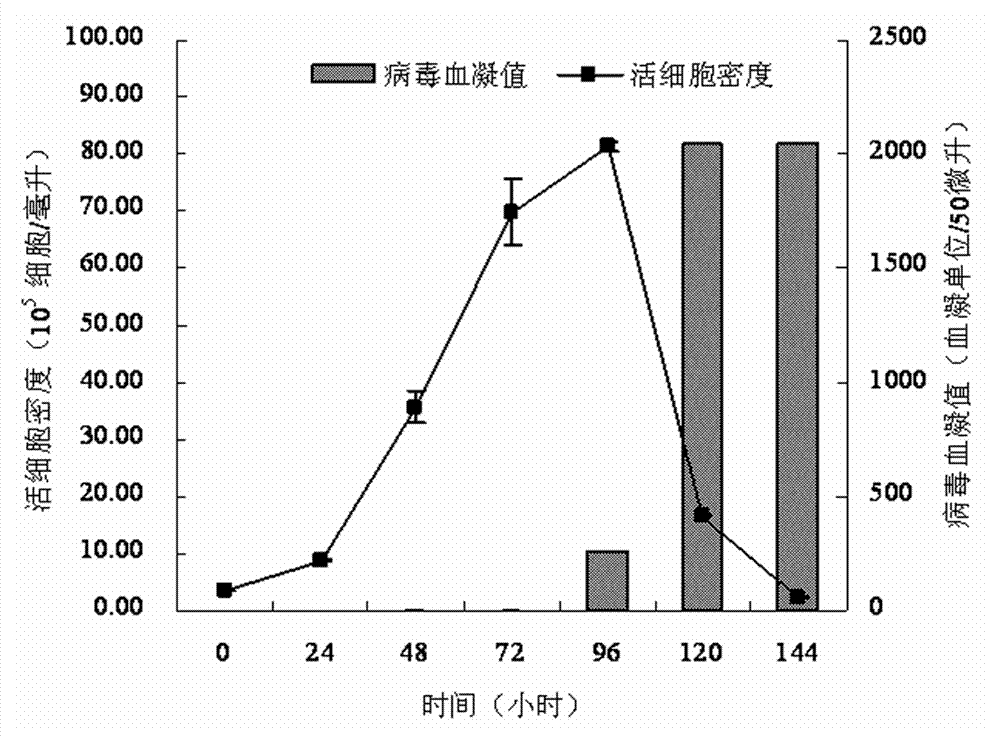
[0049] A serum-free medium suitable for large-scale production of influenza vaccines, containing 23 basic metabolic nutrients, 2 nucleic acid compounds, 6 vitamins, 9 inorganic salt compounds, 1 shear protectant, 2 pH buffer, 1 pH indicator, 10 virus proliferation promoters and 3 additives, dissolve the following components in pyrogen-free ultrapure water, and the various components are as follows, the unit is mg / L:
[0050] Glucose (D-Glucose) 5400 Sodium Pyruvate 110 Alanine (L-Alanine) 4.45 Arginine (L-Arginine Hydrochloride) 147.5 Asparagine (L-Asparagine Monohydrate) 7.5 Aspartic acid (L-Aspartic cid) 6.65 Cystine (L-Cystine Dihydrochloride) 17.56 Cysteine (L-Cysteine Hydrochloride Monohydrate) 31.29 Glutamic Acid (L-Glutamic Acid) 7.35 Glutamine (L-Glutamine) 876 Glycine 18.75 Histidine (L-Histidine Hydrochloride Monohydrate) 31.48 Isoleucine (L-Isoleucine) 54.47 Leucine (L-Leucine) ...
Embodiment 2
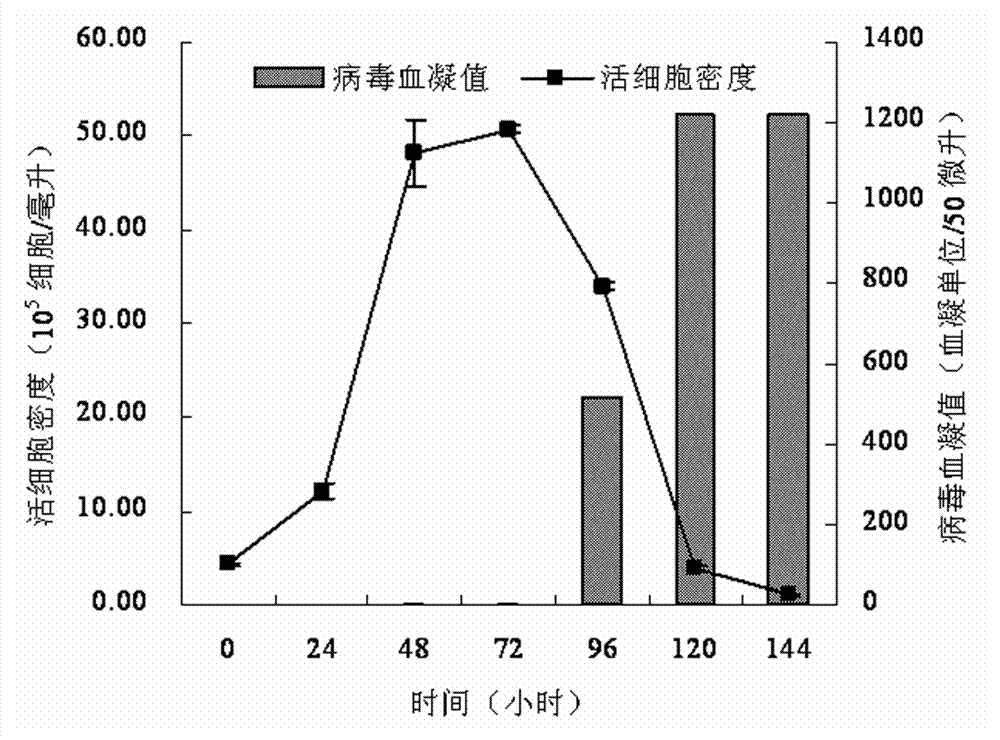
[0054] A serum-free medium suitable for large-scale production of influenza vaccines, containing 23 basic metabolic nutrients, 2 nucleic acid compounds, 6 vitamins, 9 inorganic salt compounds, 1 shear protectant, 2 pH buffer, 1 pH indicator, 10 virus proliferation promoters and 3 additives, dissolve the following components in pyrogen-free ultrapure water, and the various components are as follows, the unit is mg / L:
[0055] Glucose (D-Glucose) 1000 Sodium Pyruvate 50 Alanine (L-Alanine) 4 Arginine (L-Arginine Hydrochloride) 40 Asparagine (L-Asparagine Monohydrate) 5 Aspartic acid (L-Aspartic cid) 2 Cystine (L-Cystine Dihydrochloride) 5 Cysteine (L-Cysteine Hydrochloride Monohydrate) 10 Glutamic Acid (L-Glutamic Acid) 3 Glutamine (L-Glutamine) 100 Glycine 5 Histidine (L-Histidine Hydrochloride Monohydrate) 10 Isoleucine (L-Isoleucine) 20 Leucine (L-Leucine) 30 Lysine (L-Lydine hydroc...
Embodiment 3
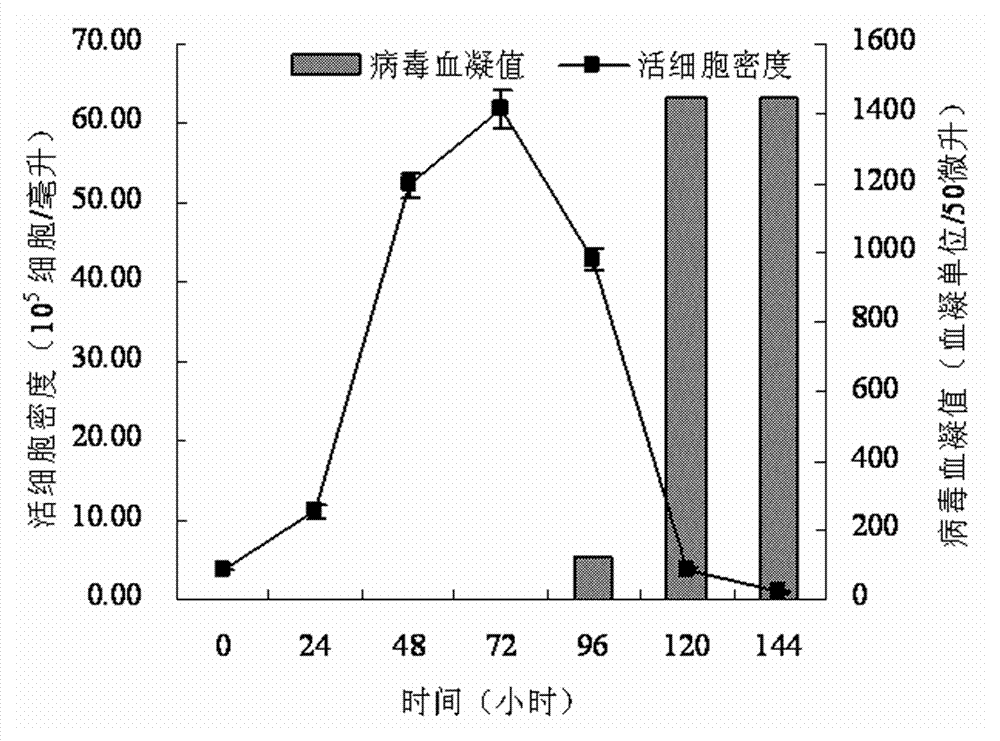
[0059] A serum-free medium suitable for large-scale production of influenza vaccines, containing 23 basic metabolic nutrients, 2 nucleic acid compounds, 6 vitamins, 9 inorganic salt compounds, 1 shear protectant, 2 pH buffer, 1 pH indicator, 10 virus proliferation promoters and 3 additives, dissolve the following components in pyrogen-free ultrapure water, and the various components are as follows, the unit is mg / L:
[0060] Glucose (D-Glucose) 6000 Sodium Pyruvate 200 Alanine (L-Alanine) 9 Arginine (L-Arginine Hydrochloride) 200 Asparagine (L-Asparagine Monohydrate) 20 Aspartic acid (L-Aspartic cid) 20 Cystine (L-Cystine Dihydrochloride) 50 Cysteine (L-Cysteine Hydrochloride Monohydrate) 50 Glutamic Acid (L-Glutamic Acid) 30 Glutamine (L-Glutamine) 2000 Glycine 50 Histidine (L-Histidine Hydrochloride Monohydrate) 50 Isoleucine (L-Isoleucine) 70 Leucine (L-Leucine) 80 Lysine (L-Lydin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com