Establishment and application of CHO cell line for producing fucose-free monoclonal antibody
A monoclonal antibody, fucus-free technology, applied in the field of bioengineering, can solve the problems of time-consuming, high cost, cumbersome operation, etc., and achieve the effect of simple operation, low time cost and effective editing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
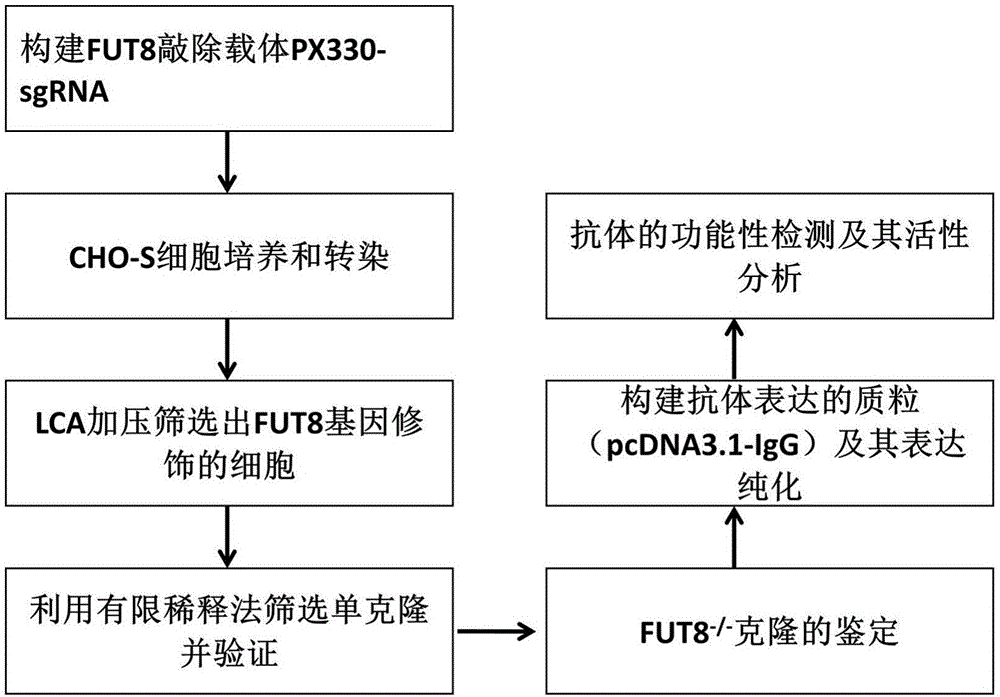
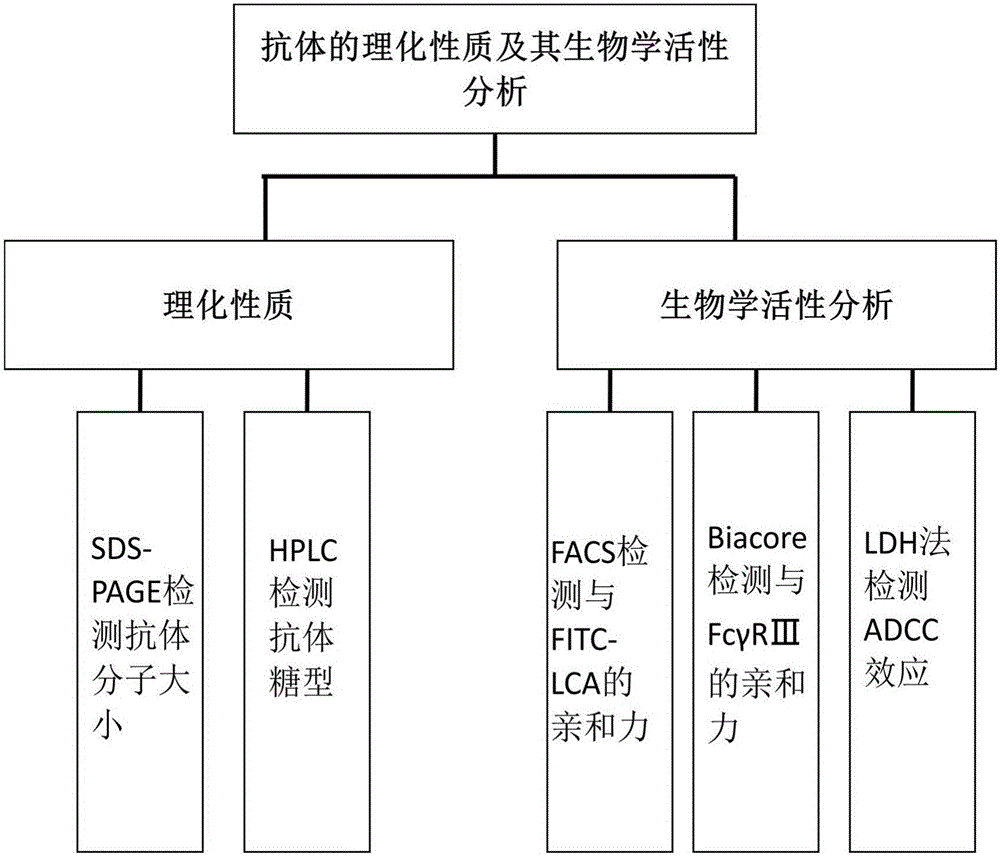
[0053] This example relates to the establishment and application of a CHO cell line producing a fucose-free monoclonal antibody; its construction process and analysis process are as follows figure 1 , 2 As shown, the specific steps are as follows:
[0054] 1: Construction of FUT8 knockout vector PX330-sgRNA plasmid
[0055] From the protein sequence (NCBI: XP_003501783.1, SEQ ID NO.12) corresponding to the FUT8 gene (its mRNA sequence (CHO-WT) is shown in SEQ ID NO.1), it can be seen that the amino acid position corresponding to exon9 has The function is the binding site of GDP-fucose, which can transfer GDP-fucose to the N-acetylglucosamine core site of the expressed protein. If the gene sequence is mutated, the function of α-1,6 fucosyltransferase can be lost.
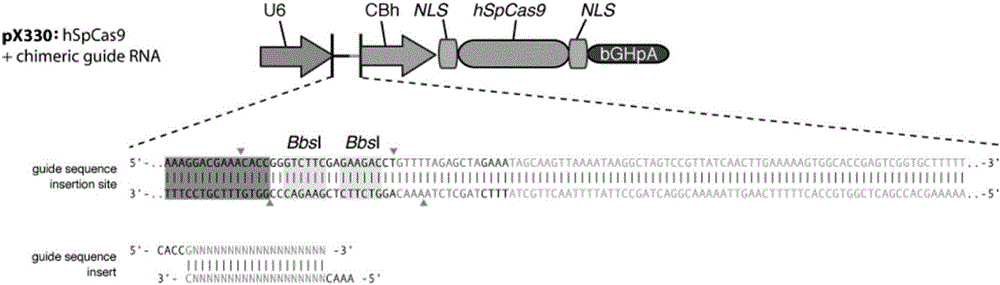
[0056] The pX330 plasmid contains human codon-optimized streptococcal hspCas9 endonuclease coding sequence and U6 RNA polymerase promoter sequence. The schematic diagram of the partial structure of the plasmid is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com