Establishing method of pig immunoglobulin Fc fragment-swine classical fever E2 fusion protein in CHO cell strain, as well as preparation method and application of fusion protein
A fusion protein and cell line technology, applied in chemical instruments and methods, biochemical equipment and methods, fusion polypeptides, etc., can solve the problems of short antibody production time, immune death, poor immune effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Embodiment 1, the acquisition of PigFC-pigSCFVE2 mutant and its coding gene
[0067] In order to improve the long-term effect of wild-type E2, the protein sequence of classical swine fever E2 was fused with porcine FC antibody fragments. The specific protein sites of specific fusion proteins are shown in Table 1.
[0068] Table 2 Fusion protein design
[0069] name PigFC-pigSCFVE2 porcine FC fragment 22-242 link peptide 243-258 E2 259-631 E2 epitope optional
[0070] Synthesis of the PigFC-pigSCFVE2 gene shown in SEQ ID No.1 is the fusion of the N-terminus of the E2 protein with the porcine FC fragment. The indicated protein PigFC-pigSCFVE2. E2 mutants can also be obtained by fusing the E2 immune epitope to the C-terminus of the E2 protein and keeping the amino acid residues of E2 unchanged.
Embodiment 2
[0071] Embodiment 2, the preparation of E2 expression antigen
[0072] 1. Construction of recombinant PigFC-pigSCFVE2 recombinant expression vector
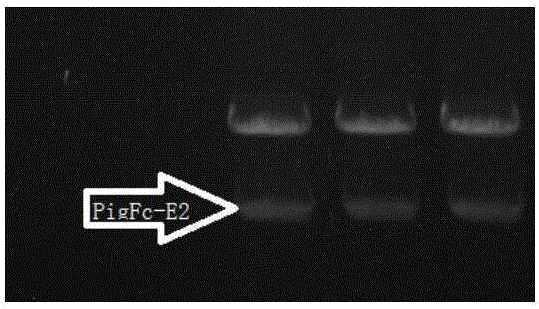
[0073] Use NheI and XhoI to double digest the PMD18-PigFC-pigSCFVE2 carrier to obtain the DNA synthesis DNA nucleic acid molecule shown in SEQ ID No.1. The fragment of about 2000bp is the target fragment, which contains the DNA fragment of the coding gene of PigFC-pigSCFVE2 or the mutation of the coding gene DNA fragments were recovered and purified. The result is as figure 1 .
[0074] The purified DNA fragment containing the coding gene of PigFC-pigSCFVE2 was digested with NheI and XhoI double-digestion eukaryotic expression vector pcCND3.1(-) respectively, the effect is as follows figure 2 , the synthetic gene was ligated to the vector segment (the ligation system was 0.5 μl pcCND3.1(-); 4.5 μl PigFC-pigSCFVE2 digested fragment, 2xT4 quick ligase, room temperature for 3 hours). The ligation product was transformed into D...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com