Method of preparing recombinant small molecular protein or polypeptide with hirudin as fusion partner
A small molecule protein and fusion partner technology, applied in the field of recombinant polypeptide fusion expression technology, can solve the problems affecting the final yield of small molecule target protein and low ratio of small molecule target protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Design and Cloning of Hirudin III (HV3) Fusion Tag and Target Polypeptide Lunasin (Lunasin) Fusion Gene
[0022] Hirudin III (HV3) fusion tag and target polypeptide Lunasin (Lunasin) fusion protein includes (from N-terminal to C-terminal): (1) 66 amino acid residues of hirudin III (HV3); (2) -GGGDDDDK- Connecting peptide (where DDDDK is the cleavage site of enterokinase); (3) 43 amino acid residues of Lunasin. On this basis, the above-mentioned fusion protein encoding gene was designed and synthesized according to E.coli preferred codons (see figure 1 ).
Embodiment 2
[0023] Example 2 Construction of Hirudin III-Lunasin Fusion Protein Expression Strain
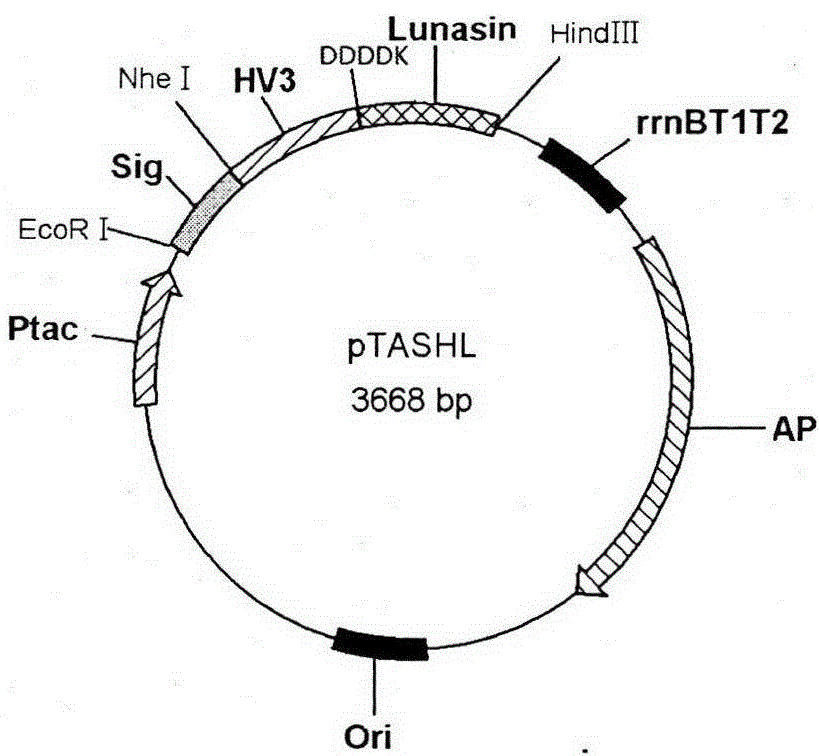
[0024] The fusion gene described in Example 1 was digested with Nhe I and Hind III and then subcloned into the expression vector pTASH (Tan S, Wu W, Liu J, Protein Expr Purif2002, 25, 430-436.) site, the resulting recombinant expression vector was named pTASHL (see figure 2 ), and sequenced to verify its correctness. with CaCl 2 The recombinant expression plasmid pTASHL was transformed into E.coli JM109 host bacteria to obtain rHV3-Lunasin fusion protein expression engineering bacteria JM109 / pTASHL.
Embodiment 3
[0025] Example 3 Expression of Hirudin III-Lunasin Fusion Protein in 7L Reactor
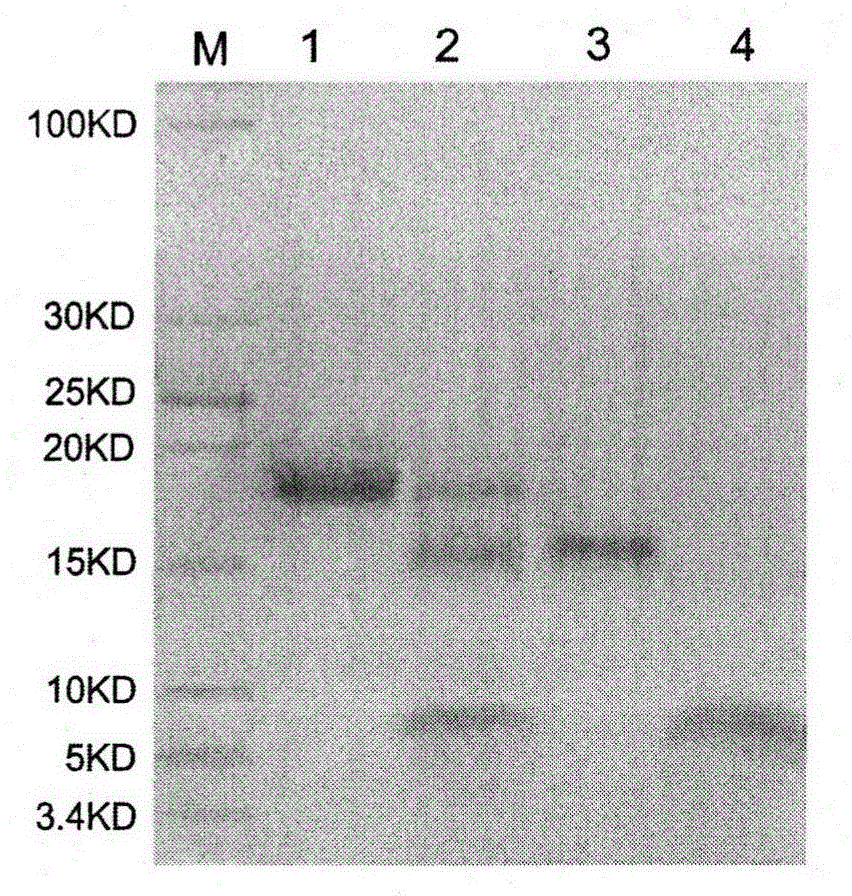
[0026]Inoculate a single colony of JM109 / pTASHL engineering bacteria in 200ml LB liquid medium (containing 100μg / ml ampicillin) at 37°C, 220rpm, and culture for 12h. Inoculate 5L fermentation medium (1% tryptone, 0.5% yeast powder, 4% sodium glutamate, 1% malt Powder, 0.671%KH 2 PO 4 , 0.757% Na 2 HPO 4 · 12H2O, 100 μg / ml ampicillin, pH 6.5), 37 ° C stirring culture, the pH of the fermentation broth is controlled at 6.5-7.2 with phosphoric acid, and the dissolved oxygen is controlled at 40%-60%. When the fermentation broth OD 600nm When reaching 3.0 with about 30ml h -1 l -1 Feed medium I (10% malt powder, pH 6.5), the feeding time is maintained for about 4 hours, and the growth of the bacteria reaches the plateau stage. -1 l -1 Feed medium II (3.33% peptone, 1.67% yeast powder, 13.3% sodium glutamate, 10% malt powder, pH6.5) until the end of fermentation. The whole fermentation process...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Apparent molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com