Cell culture method for reducing acidic peak content of antibody and improving glycoform of antibody
A cell culture and antibody technology, applied in the field of cell culture, to achieve the effect of ensuring drug efficacy and optimizing glycosylation level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Culture of a cell line A expressing anti-tumor necrosis factor-α (TNFα) antibody.
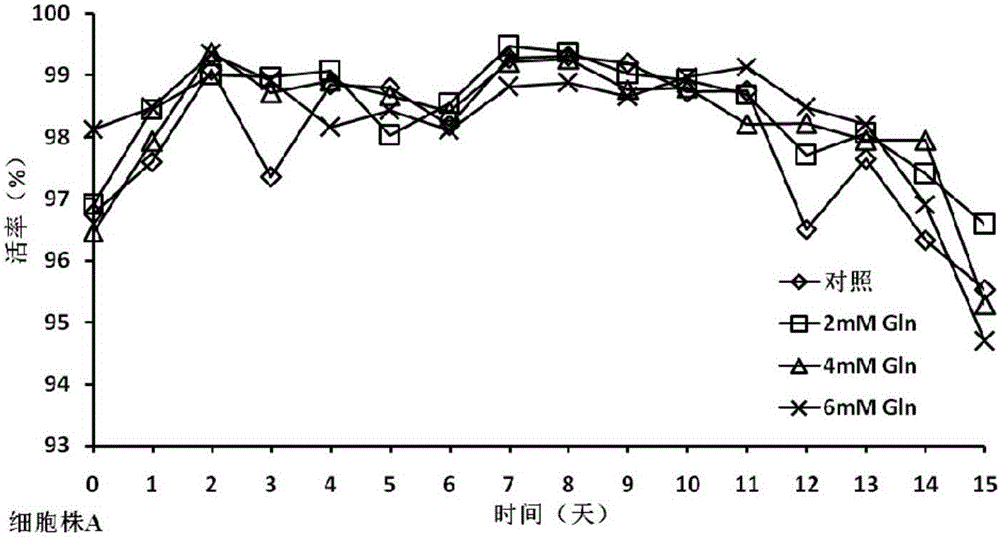
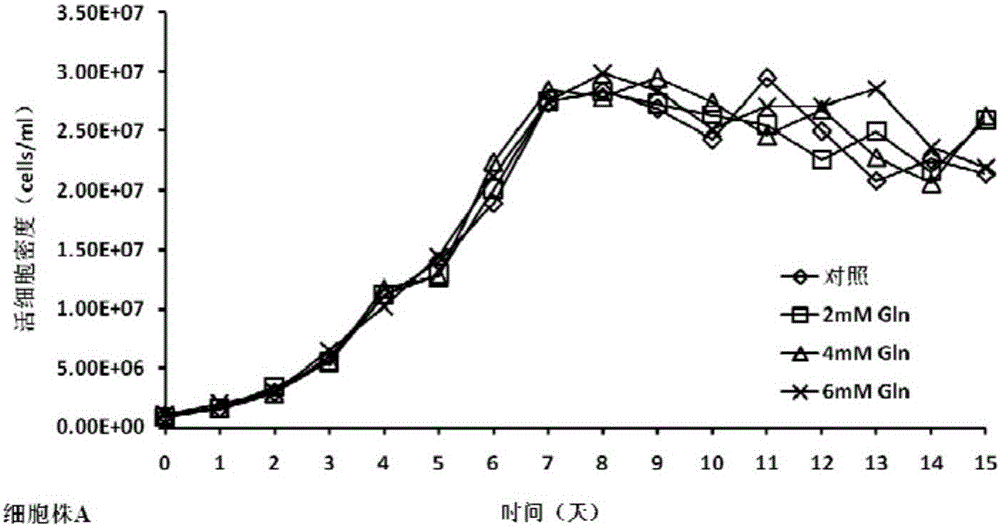
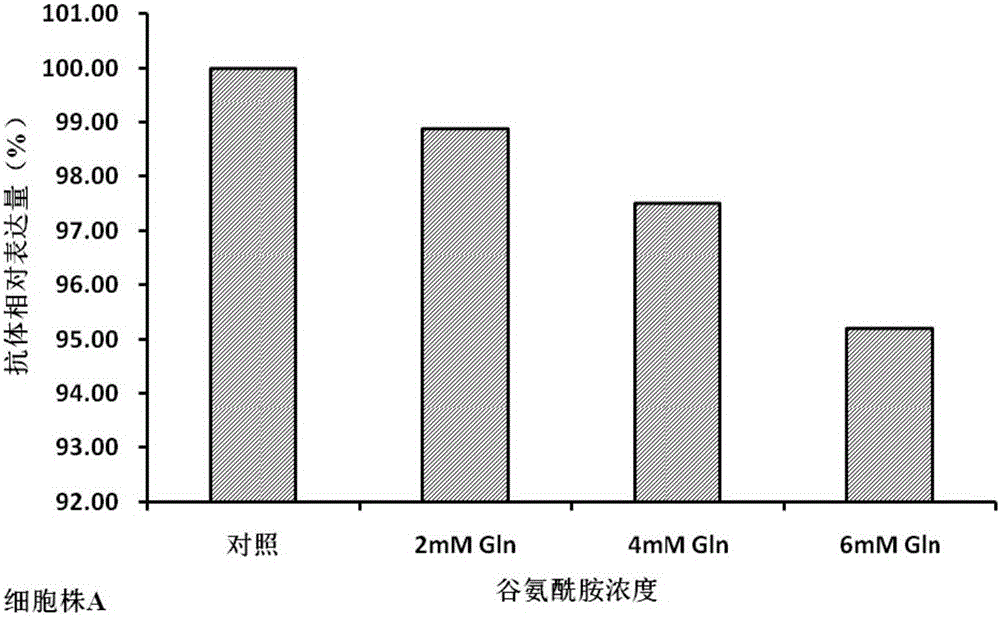
[0034] In the cell culture experiment, on the 0th day, the basal medium CDM4PERMAb with the final concentrations of 0mM (control group), 2mM, 4mM, and 6mM glutamine were all added to 500ml shake flasks respectively, and the cells were divided into 1×10 6 Cells / mL were inoculated and cultured at 37°C. From the third day, the cell viability and density and various biochemical parameters in the culture were measured every day, and fed-batch culture was carried out. The fed-batch medium was CHOCDEfficientFeed TM A, According to the results detected by the biochemical analyzer, the concentration of glucose was controlled at 5 g / L, the concentration of monosodium glutamate at 4 mM, the temperature was controlled at 33 ° C from the 6th day, and the harvest was performed on the 15th day of cell culture.
[0035] The cell culture process involves detection of cell viability and density, concentra...
Embodiment 2
[0051] Cell line B was used for expression and culture of recombinant human tumor necrosis factor receptor-Fc recombinant protein (rhTNFR-Fc) antibody.
[0052] In the cell culture experiment, on the 0th day, the basal medium CDFortiCHOAGT with the final concentrations of 0mM (control group), 2mM, 4mM, and 6mM glutamine were all added to 500ml shake flasks, and the cells were divided into 1×10 6 Cells / mL was inoculated and cultured at 37°C. From the third day, the cell viability and density and various biochemical parameters in the culture were measured every day, and fed-batch culture was carried out. The fed-batch medium was EfficientFeed TM B+, according to the results detected by the biochemical analyzer, the glucose concentration was controlled at 10g / L, the monosodium glutamic acid concentration was 8mM, the temperature was controlled at 34°C from the sixth day, and the cells were harvested on the 13th day of cell culture. The antibody quality detection method is the sam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com