A cell culture method for reducing antibody acidic peak content and improving antibody glycoform
A cell culture and antibody technology, applied in the field of cell culture, to achieve the effect of ensuring drug efficacy and optimizing glycosylation level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Cultivation of a cell line A expressing antibodies against tumor necrosis factor-α (TNFα).
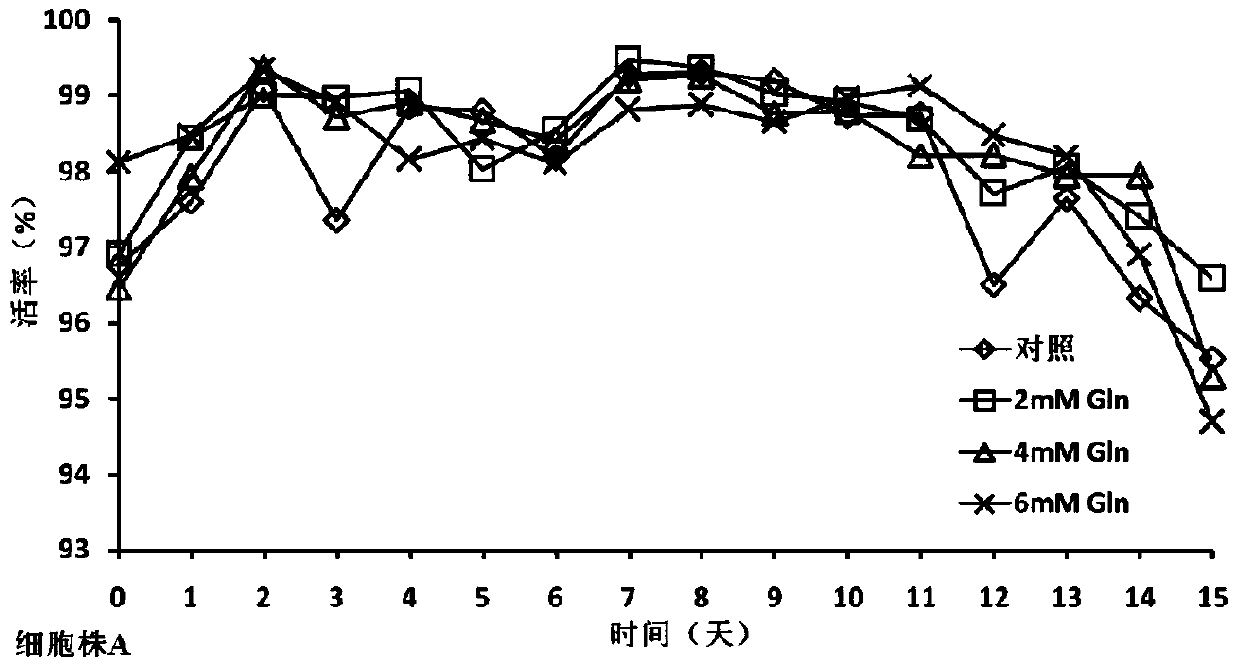
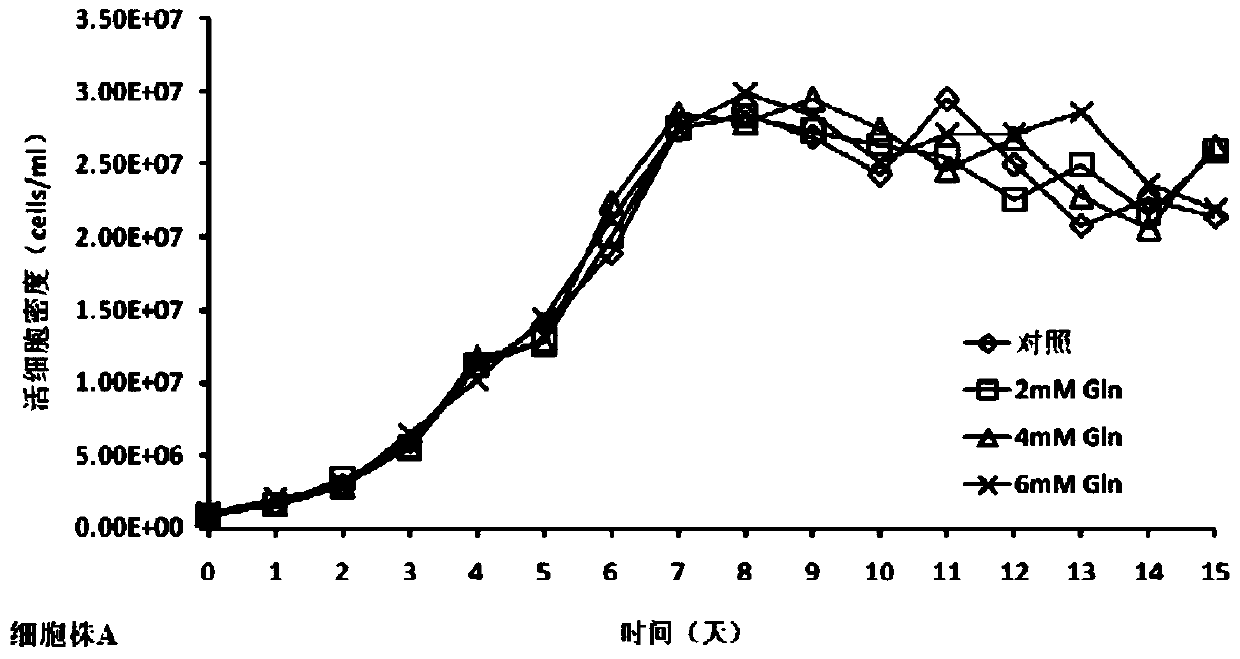
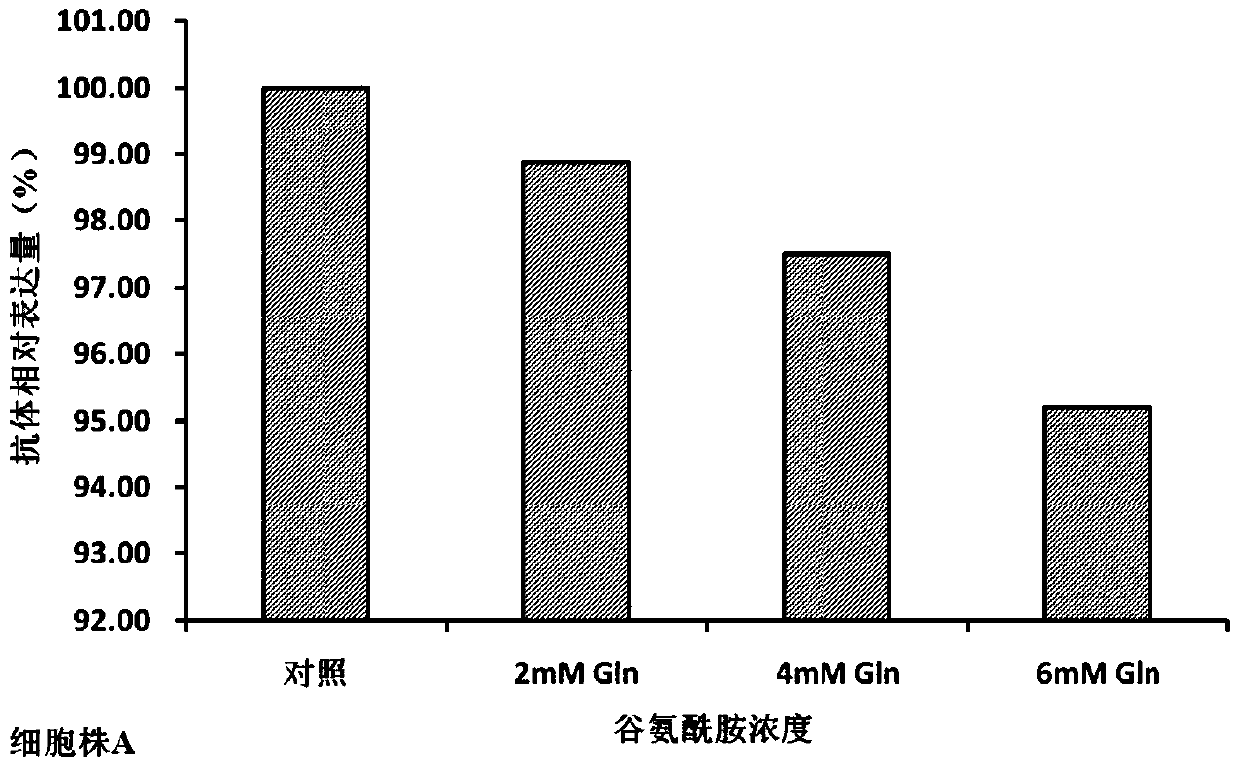
[0034] In the cell culture experiment, on day 0, the basal medium CDM4PERMAb with final concentrations of 0mM (control group), 2mM, 4mM, and 6mM glutamine were added to 500ml shake flasks. The cells were divided into 1×10 6 Cells / mL were inoculated and cultured at 37°C. From day 3, cell viability and density and various biochemical parameters in the culture were measured every day, and fed culture was carried out. The fed culture medium was CHO CD Efficient Feed TM A. Control the glucose concentration at 5g / L and the monosodium glutamate concentration at 4mM according to the results of the biochemical analyzer, and control the temperature at 33°C on the 6th day, and harvest on the 15th day of cell culture.
[0035] The cell culture process involves the detection of cell viability and density, anti-TNFα antibody concentration, anti-TNFα antibody charge heterogeneity, anti-TNFα antibody...
Embodiment 2
[0051] Cell line B was cultured for expression of recombinant human tumor necrosis factor receptor-Fc recombinant protein (rhTNFR-Fc) antibody.
[0052] In the cell culture experiment, on day 0, the basal medium CD Forti CHO AGT with final concentrations of 0 mM (control group), 2 mM, 4 mM, and 6 mM glutamine were added to 500 ml shake flasks. 10 6 Cells / mL were inoculated and cultured at 37°C. From the third day on, the cell viability and density and various biochemical parameters in the culture were measured every day, and fed culture was carried out. The fed culture medium was Efficient Feed TM B+, according to the results of the biochemical analyzer, the glucose concentration was controlled at 10g / L, the monosodium glutamate concentration was at 8mM, the temperature was controlled at 34°C on the sixth day, and the cell culture was harvested on the 13th day. The antibody quality detection method is the same as in Example 1. The cell viability, viable cell density, antibody exp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com