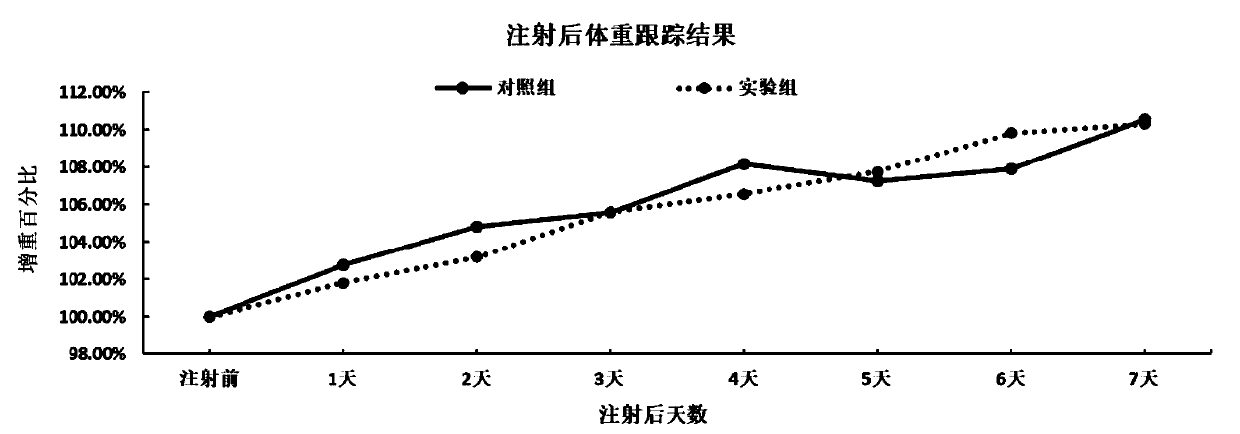
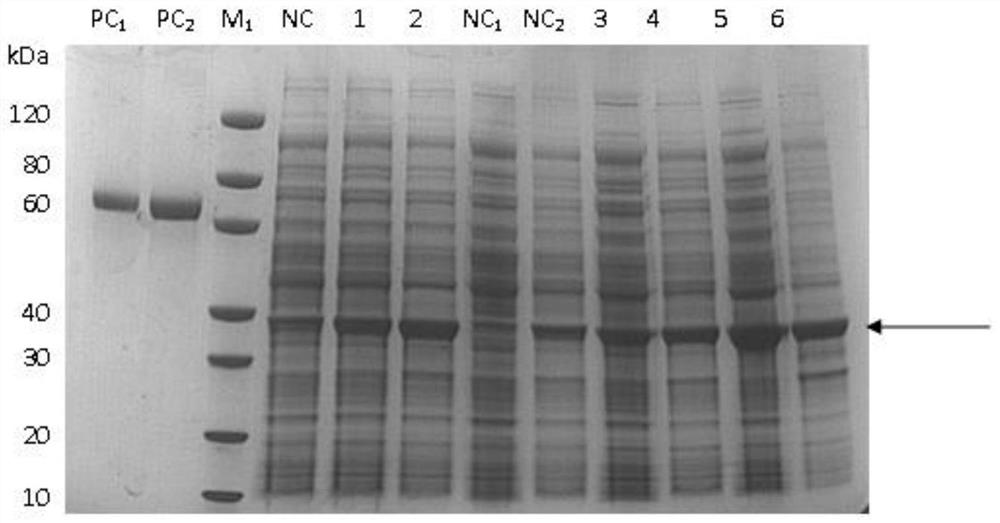
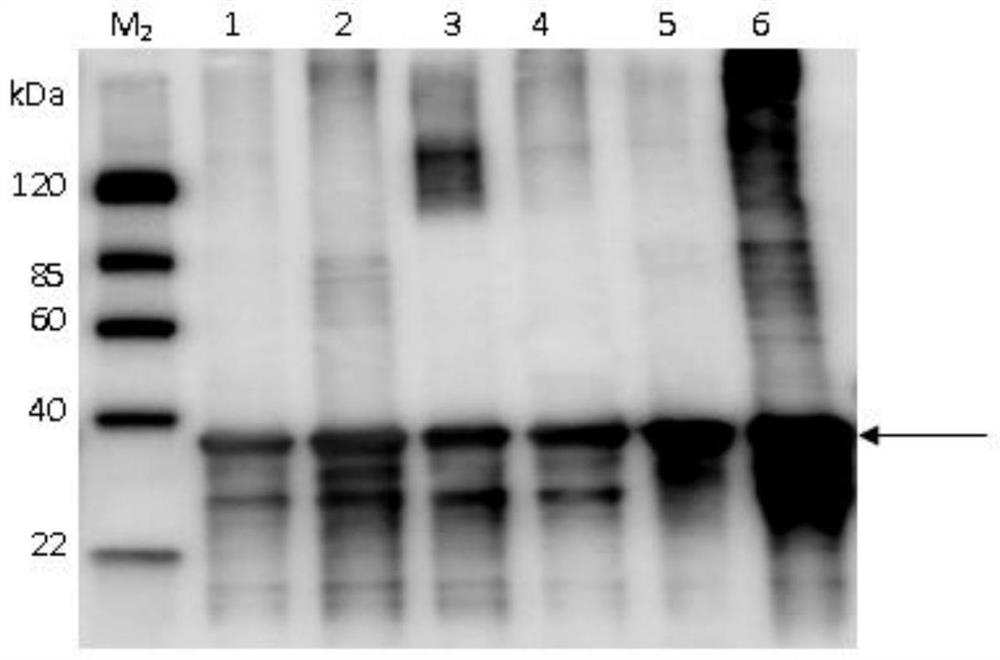
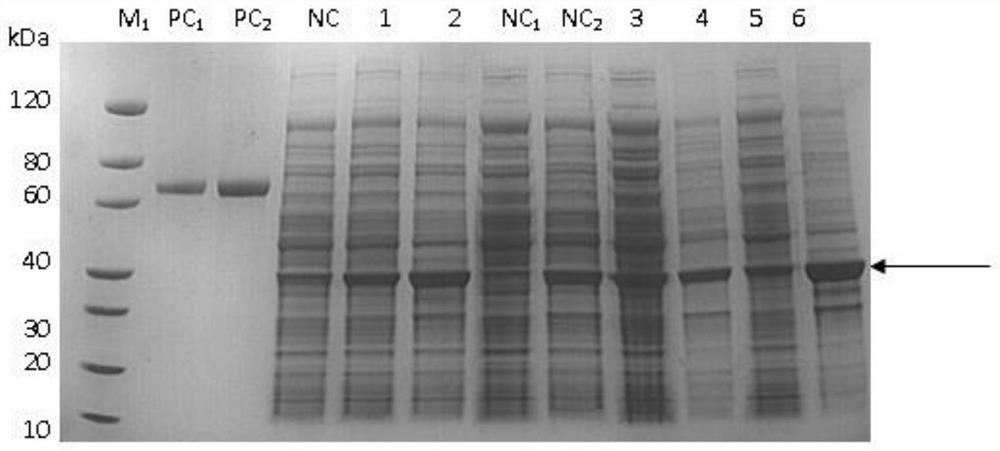
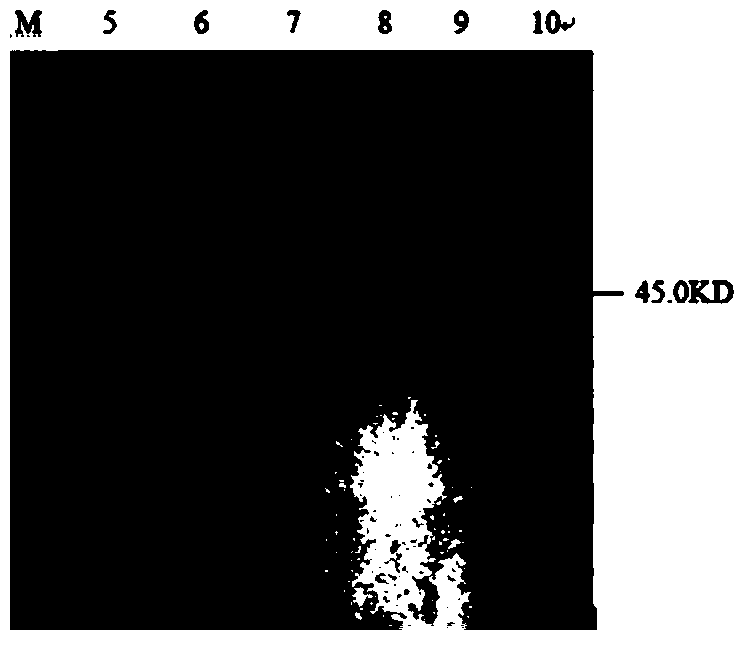Patents
Literature
44 results about "Clostridium perfringens toxoid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Clostridium perfringens alpha toxin is a toxin produced by the bacterium Clostridium perfringens (C. perfringens) and is responsible for gas gangrene and myonecrosis in infected tissues.
Clostridium perfringens alpha toxin double-antibody sandwich ELIS quantitative determination method
ActiveCN103941004AStrong specificityStrong responsivenessMaterial analysisAlpha-toxinClostridium perfringens toxoid
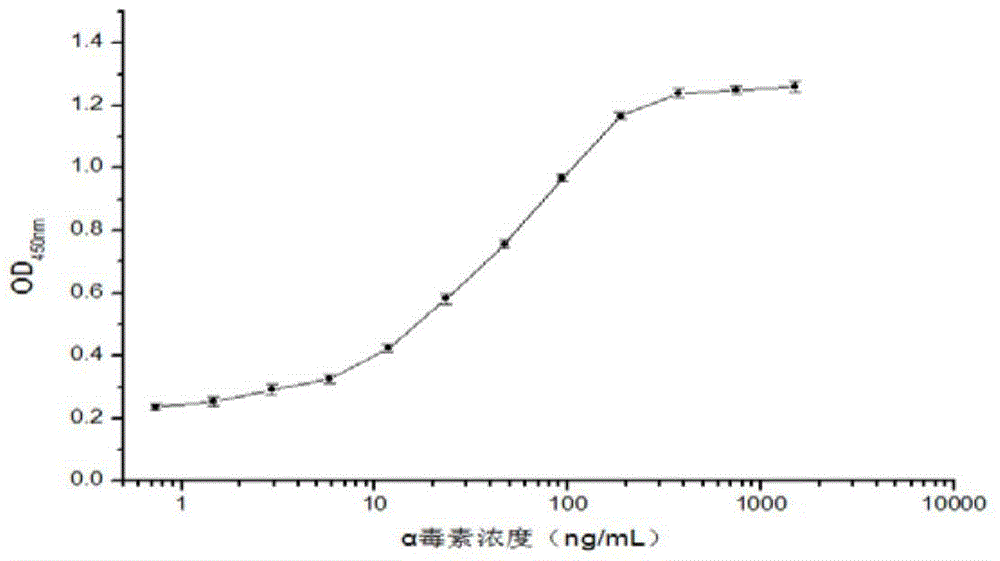
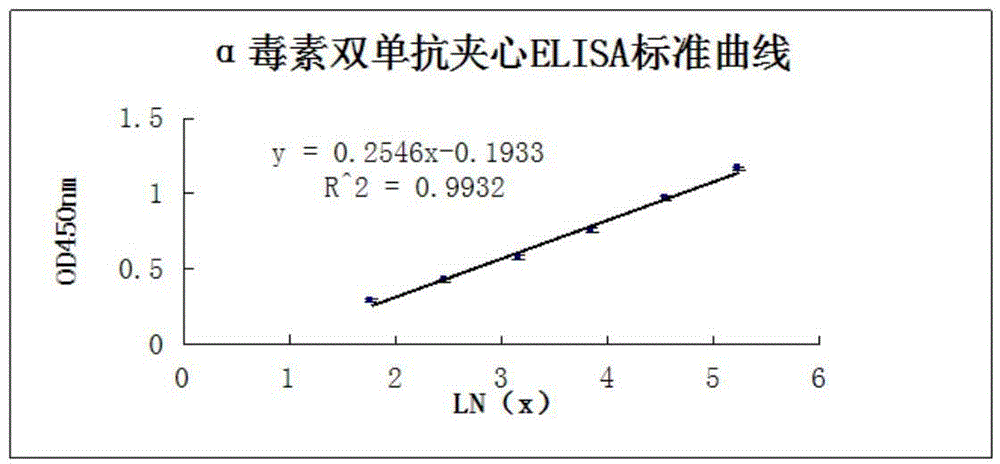
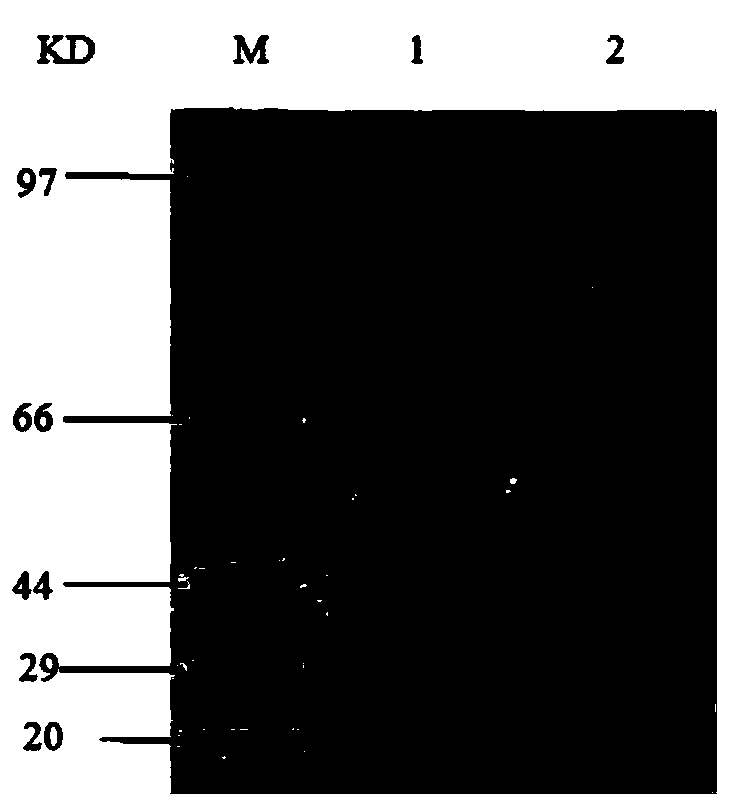
The invention relates to a clostridium perfringens alpha toxin double-antibody sandwich ELIS quantitative determination method. According to the clostridium perfringens alpha toxin double-antibody sandwich ELIS quantitative determination method, alpha toxin protein obtained via prokaryotic expression is taken as an immunogen; monoclonal antibodies obtained via hybridoma technique are taken as detection antibodies and capture antibodies; reaction conditions are optimized via experiments; alpha toxin protein samples with a series of concentration are used for construction of a standard curve; the double-antibody sandwich ELIS method is established; and indexes of the double-antibody sandwich ELIS are verified. The clostridium perfringens alpha toxin double-antibody sandwich ELIS quantitative determination method specifically comprises following steps: (1) prokaryotic expression of alpha toxin; (2) preparation of anti-alpha toxin monoclonal antibodies; (3) establishment of the double-antibody sandwich ELIS method; (4) establishment of the standard curve; and (5) performance evaluation on the double-antibody sandwich ELIS method. The clostridium perfringens alpha toxin double-antibody sandwich ELIS quantitative determination method is excellent in specificity, and high in sensitivity and stability, is fast and convenient, and can be used for effective quantitative determination of alpha toxin in A-E type clostridium perfringens cultural supernatants; and the high-efficient detection method is provided for alpha toxin determination.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Monoclonal antibody blocking ELISA (enzyme-linked immuno sorbent assay) testing method of clostridium perfringens alpha toxin
The invention discloses a monoclonal antibody blocking ELISA testing method of a clostridium perfringens alpha toxin, which is used for early testing of the alpha toxin and provides an effective testing tool for early diagnosis, spreading prevention and subsequent research of clostridium perfringens. The method comprises the following two steps: I, establishing the monoclonal antibody blocking ELISA testing method; and II, determining a result judgment criterion. The monoclonal antibody blocking ELISA testing method which has good specificity and stability and high sensitivity, is quick, simple and convenient, and tests the alpha toxin efficiently is established for the first time, and the method is simple to implement and high in testing speed and can achieve quantitative testing and massive testing. In a word, monoclonal antibody blocking ELISA is expected to play an important role in prevention and treatment of clostridium perfringens diseases.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Bovine type-A clostridium perfringens subunit vaccine and preparation method and application thereof
ActiveCN107596361AStrong immune responseOvercoming the problem of incomplete inactivationAntibacterial agentsBacterial antigen ingredientsAntigenAlpha-toxin
The invention provides a bovine type-A clostridium perfringens subunit vaccine and a preparation method and an application thereof. The subunit vaccine comprises a bovine type-A clostridium perfringens alpha-toxin protein truncation body and a pharmacologically acceptable adjuvant. The bovine type-A clostridium perfringens alpha-toxin protein truncation body is an antigen determinant amino acid sequence from 255th to 372nd amino acids of alpha-toxin protein. The subunit vaccine has the following advantages that 1) safety problems due to uncompleted inactivation are not existed; 2) the qualityis controllable, and the difference between the batches is not existed; 3) the production equipment and space requirement is low, the expression level is high, and the cost is low; and 4) toxin dispersion risk is not existed, and the safety of operators can be guaranteed.
Owner:NOVO BIOTECH CORP
Clostridium perfringens alpha, beta 1, beta 2 and epsilon coexpression vector and construction method and expression method thereof
InactiveCN104131022AAvoid interactionStable expressionVector-based foreign material introductionBacillus perfringensGreek letter epsilon
The invention discloses a clostridium perfringens alpha, beta 1, beta 2 and epsilon toxin coexpression vector; vectors are respectively pETDuet-1 and pRSFDuet-1 expressed in the same host bacteria, and the vectors are respectively connected with clostridium perfringens alpha and epsilon toxin encoding genes and clostridium perfringens beta 1 and beta 2 toxin encoding genes. On the basis, the invention also discloses a construction method and an expression method of the coexpression vector.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
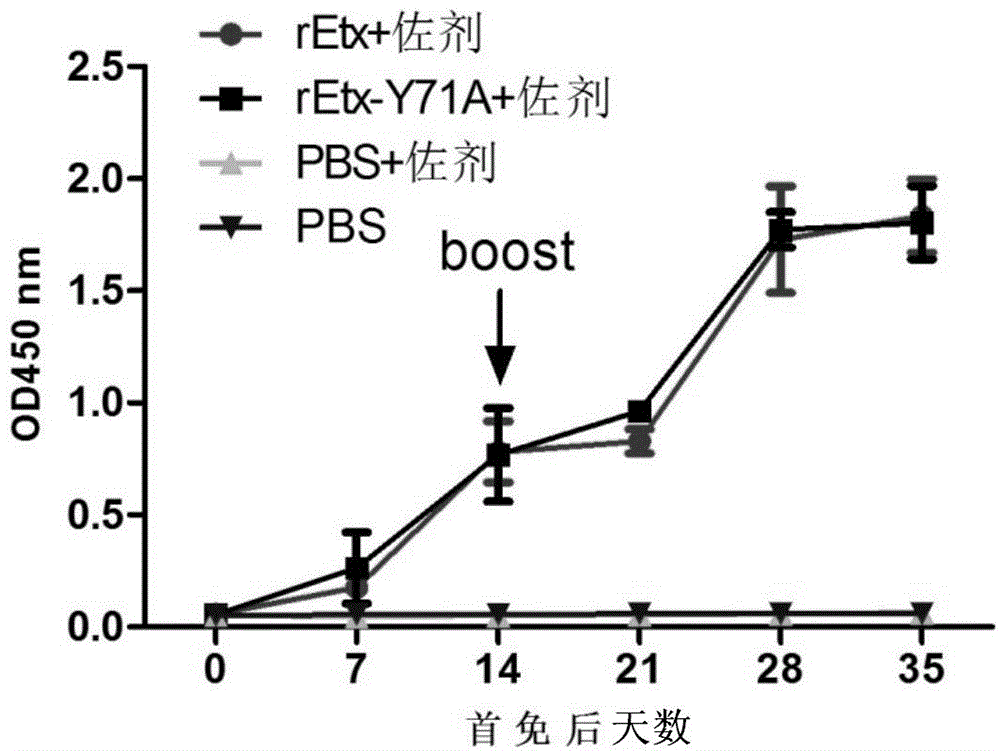
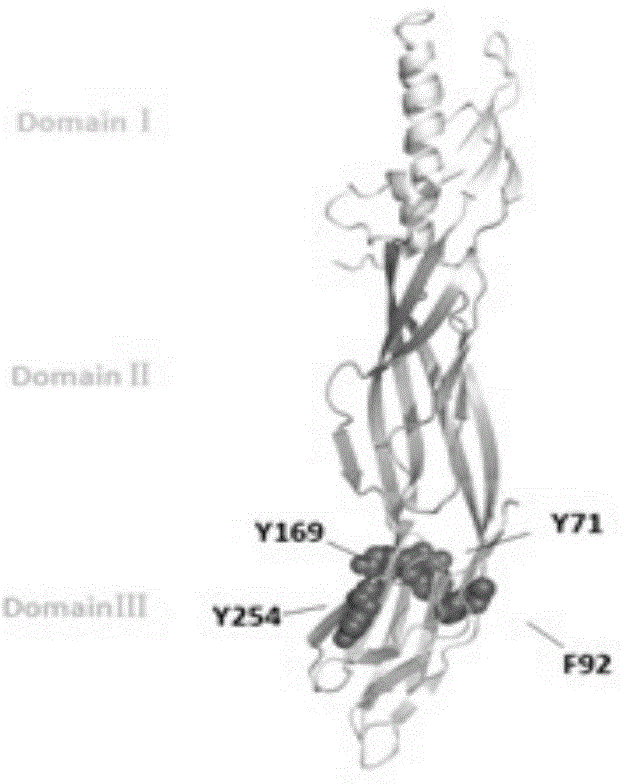
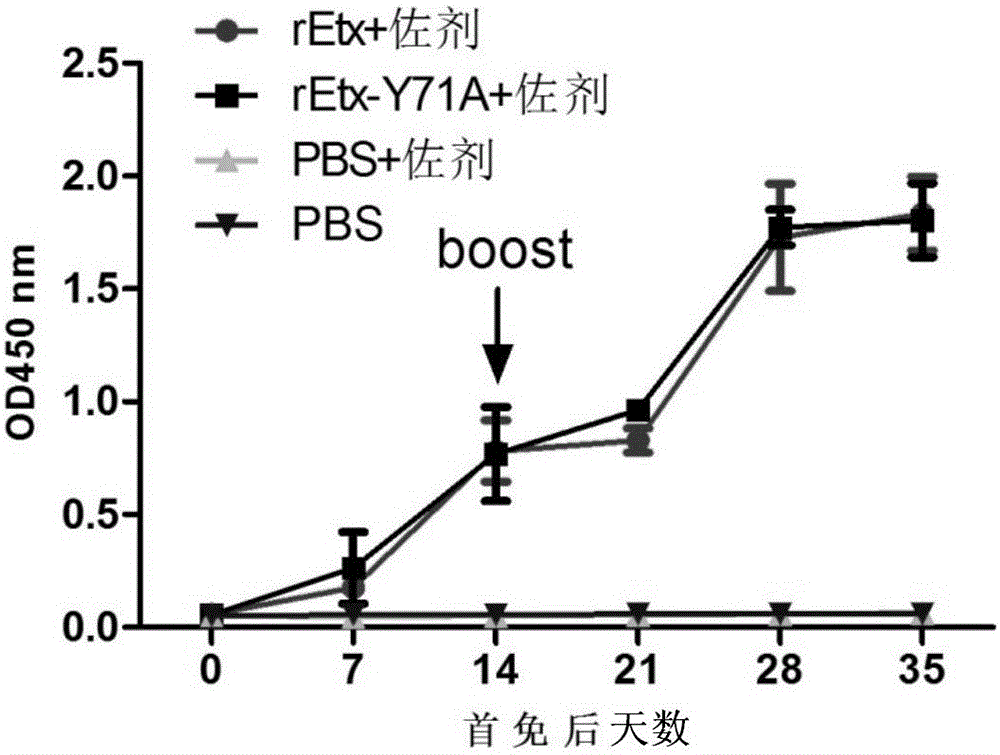
Toxin attenuation mutant for epsilon toxin of clostridium perfringens and application of toxin attenuation mutant
ActiveCN104560780AGood immunogenicity and immunoprotectionAchieve complete attenuationAntibacterial agentsBacterial antigen ingredientsAromatic amino acidsMutant
The invention discloses a toxin attenuation mutant for epsilon toxin of clostridium perfringens and an application of the toxin attenuation mutant for the epsilon toxin of the clostridium perfringens. An epsilon toxin mutant for attenuating toxin in cells or animals is obtained by mutating tyrosine at a site 71 of mature toxin of the epsilon toxin of wide clostridium perfringens into non-aromatic amino acids. The invention further discloses a recombinant expression vector and a recombinant host cell which contain coding genes of the toxin attenuation mutant for the epsilon toxin of the clostridium perfringens. According to the recombined and expressed toxin attenuation mutant for the epsilon toxin of the clostridium perfringens, the complete toxin attenuation of mice in vitro and in vivo can be achieved, and good immunogenicity and immunizing protection are presented in a mouse model. The toxin attenuation mutant for the epsilon toxin of the clostridium perfringens and the coding genes of the toxin attenuation mutant can be used for preparing subunit vaccines of the epsilon toxin of the clostridium perfringens or subunit vaccines of multivalent clostridium toxin.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Genetically engineered vaccine of epsilon toxin of clostridium perfringens and application thereof
InactiveCN103830747AImprove immunityNo side effectsAntibacterial agentsBacterial antigen ingredientsDiseaseChinchilla
The invention discloses a genetically engineered vaccine of epsilon toxin of clostridium perfringens and an application thereof. The genetically engineered vaccine of epsilon toxin of clostridium perfringens is obtained by the following steps: firstly, designing a specific primer; carrying out PCR (Polymerase Chain Reaction) amplification to obtain an epsilon gene segment, and cloning to a pMD18-T Vector; selecting a positive recombinant, and guiding a recombinant plasmid into an acceptor strain to successfully construct a recombinant strain BL21 (DE3)-epsilon; and finally, emulsifying the prepared epsilon-toxin protein and an aluminum hydroxide adjuvant in a volume ratio of 9:1, and adding 0.01% of thiomersalate to obtain the genetically engineered vaccine of epsilon toxin of clostridium perfringens. The genetically engineered vaccine of epsilon toxin of clostridium perfringens can be put into industrial production easily, simple to operate, has good safety and the like, and can be applied to preventing fetal intestinal diseases of animal such as lambs, sheep, goats, calves and chinchilla, caused by D type clostridium perfringens.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Humanized single-chain antibody 8B of clostridium perfringens alpha-toxin
ActiveCN104531730AAchieve therapeutic effectExempt from humanizationAntinoxious agentsAntibody ingredientsPhage antibodiesSingle-Chain Antibodies
The invention discloses a humanized single-chain antibody 8B of clostridium perfringens alpha-toxin. The humanized single-chain antibody 8B is screened from a phage antibody library Source bioscience, is a full-humanized antibody of the clostridium perfringens alpha-toxin and can be used for achieving a toxin neutralization treating effect, overcoming various side effects of a heterologous antibody and eliminating complex steps and high cost of humanization modification on the heterologous antibody; and the single-chain antibody is small in molecular weight, strong in in-vivo penetrability and capable of rapidly reaching to damaged tissues and cells to exert an antitoxin effect, so that the double aims of economy and high efficiency are achieved. The alpha-toxin has a certain homology with other types of toxins of clostridium perfringens, so that the antibody drug possibly generates inhibiting and treating effects for other types of toxins and can also be used as a detecting reagent to detect the clostridium perfringens alpha-toxin.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Clostridium perfringen alpha toxin genetic engineering vaccine and application thereof
InactiveCN103861094AImprove immunityTargeted optimizationAntibacterial agentsBacterial antigen ingredientsDiseaseAlpha-toxin
The invention relates to a clostridium perfringen alpha toxin genetic engineering vaccine and application thereof. The clostridium perfringen alpha toxin genetic engineering vaccine is prepared by designing a specific primer, implementing polymerase chain reaction (PCR) amplification on A-type clostridium perfringen DNA to obtain an alpha toxin gene segment, cloning the gene segment onto a pMD18-T Vector, selecting a positive recon and linking the positive recon onto a PET-28 alpha (+) plasmid; introducing the recombinant plasmid to a receptor strain to successfully construct a recombinant strain BL21 (DE3)-alpha; emulsifying the prepared alpha toxin protein and an aluminum hydroxide adjuvant at a volume ratio of 9:1, and adding 0.01% thiomersal to obtain the clostridium perfringen alpha toxin genetic engineering vaccine. The clostridium perfringen alpha toxin genetic engineering vaccine is easy for realization of industrial production, simple to operate and good in safety, and is capable of effectively preventing such diseases as animal necrotic enteritis, enterotoxemia, and human and animal traumatic gas gangrene caused by the A-type clostridium perfringens.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Clostridium perfringens type B exotoxin, preparation method thereof, toxigenic culture medium and application of clostridium perfringens type B exotoxin
ActiveCN109554420AToxic performance is stableStabilize Toxic EffectsAntibacterial agentsBacterial antigen ingredientsVaccine ProductionBacillus perfringens
The invention discloses a clostridium perfringens type B exotoxin, a preparation method thereof, a toxigenic culture medium and an application thereof. Each 1000 ml of the toxigenic culture medium isprepared from the following raw materials by weight: 15-25 g of peptone, 3-5 g of yeast extract, 3-4 g of NaCl, 5-10 g of Na2HPO4.12H2O, 5-10 g of dextrin or 5-10 g of coarse dextrin, and the balanceof water for injection. The virulence of the clostridium perfringens toxin liquid produced by the method according to the present invention is up to 1 mouse MLD of no more than 0.0006 mL, and the virulence is 3.3 times than the vaccine production standard. Moreover, a triple inactivated vaccine against bradsot, struck (or lamb dysentery) and enterotoxemia prepared by the clostridium perfringens type B exotoxin has serum neutralizing titer in rabbits and sheep equal to or higher than the relevant standard.
Owner:JINYUBAOLING BIO PHARM CO LTD
Clostridium perfringen alpha toxin genetic engineering vaccine and preparation method thereof
ActiveCN109602898APreserve immunogenicityReduce manufacturing costAntibacterial agentsBacterial antigen ingredientsAlpha-toxinWild type
The invention provides a clostridium perfringen alpha toxin genetic engineering vaccine and a preparation method thereof. Compared with wild type alpha toxins, clostridium perfringen alpha toxin recombination protein is characterized in that the 176th site histidine of an amino acid sequence is mutated to obtain asparagine. The clostridium perfringen alpha toxin genetic engineering vaccine is prepared from detoxifcation clostridium perfringen alpha toxin recombination protein of self-induction secretory expression. The vaccine has the advantages of being safer, better in immune efficacy, simpler in technology, lower in cost and the like, and can effectively solve the problems that in the prior art, the production technology of the vaccine is complexer, and the cost is higher.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Porcine epidemic diarrhea-porcine clostridial enteritis bivalent subunit oral vaccine and preparation method thereof
PendingCN110882384AImprove immunityReduce immune stressAntibacterial agentsBacterial antigen ingredientsClostridium perfringens beta toxinClostridium perfringens toxoid
The invention belongs to the technical field of biology and provides a porcine epidemic diarrhea-porcine clostridial enteritis bivalent subunit oral vaccine and a preparation method thereof. The porcine epidemic diarrhea-porcine clostridial enteritis bivalent subunit oral vaccine comprises two antigens, a vaccine adjuvant and an oral adjuvant, wherein the two antigens are an inactivated porcine epidemic diarrhea virus S protein and an inactivated porcine clostridium perfringens beta toxin protein respectively. With the vaccine adopted, a pig can simultaneously obtain the porcine epidemic diarrhea virus antibody and the porcine clostridium perfringens beta toxin antibody, so that the pig can be prevented from being infected with epidemic diarrhea and clostridial enteritis. The vaccine is taken orally, so that an immunization route is changed; the immunological stress of the pig can be reduced; the side effect of the vaccine is slight; and therefore, the immune effect of the vaccine is effectively improved. The preparation method of the oral vaccine provided by the invention is simple and easy to operate and does not need special production equipment. The production cost of the oralvaccine is lower.
Owner:天康生物制药有限公司
Humanized single-chain antibody 7D of clostridium perfringens alpha-toxin
ActiveCN104531731AAchieve therapeutic effectExempt from humanizationAntinoxious agentsAntibody ingredientsPhage antibodiesSingle-Chain Antibodies
The invention discloses a humanized single-chain antibody 7D of clostridium perfringens alpha-toxin. The humanized single-chain antibody 7D is screened from a phage antibody library Source bioscience, is a full-humanized antibody of the clostridium perfringens alpha-toxin and can be used for achieving a toxin neutralization treating effect, overcoming various side effects of a heterologous antibody and eliminating complex steps and high cost of humanization modification on the heterologous antibody; and the single-chain antibody is small in molecular weight, strong in in-vivo penetrability and capable of rapidly reaching to damaged tissues and cells to exert an antitoxin effect, so that the double aims of economy and high efficiency are achieved. The alpha-toxin has a certain homology with other types of toxins of clostridium perfringens, so that the antibody drug possibly generates inhibiting and treating effects for other types of toxins and can also be used as a detecting reagent to detect the clostridium perfringens alpha-toxin.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Establishment and application of surface display C type clostridium perfringens alpha and beta toxin protein recombinant lactobacillus plantarum
PendingCN110295134AImprove protectionSolve the problem of extracellular transportAntibacterial agentsBacterial antigen ingredientsSurface displayBacillus perfringens
The invention relates to surface display C type clostridium perfringens alpha and beta toxin protein recombinant lactobacillus plantarum. The C type clostridium perfringens alpha toxin gene sequence,beta1 toxin gene sequence and beta2 toxin gene sequence are inserted in a genome of lactobacillus plantarum. The invention further relates to an establishment method and application of the recombinantlactobacillus plantarum. Through a PCR method, alpha, beta1 and beta2 toxin genes are successfully cloned on the whole genome of C type clostridium perfringens (CVCC 1158), a corresponding recombinant plasmid is established and electrically transferred into lactobacillus plantarum NC8, three strains of recombinant lactobacillus plantarum are obtained after induction expression, the oral mode is selected for immunizing a rat, the immune effect of recombinant bacteria is evaluated, the result shows that when the rat is immunized by orally taking the recombinant lactobacillus plantarum, the ratbody can be stimulated to generate humoral immunity and can also be stimulated to generate cellular immunity, and a good protection effect on an animal body is realized.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Indirect ELISA method of clostridium perfringens beta2 toxin antibody
PendingCN113702639AStrong specificityIncreased sensitivityMaterial analysisClostridium perfringens toxoidBacillus perfringens
The invention discloses an indirect ELISA (enzyme-linked immuno sorbent assay) detection method for a clostridium perfringens beta 2 toxin antibody. The indirect ELISA detection method comprises the following steps: step 1, establishing an indirect ELISA method for the clostridium perfringens beta 2 toxin antibody; and step 2, determining a result judgment standard. The invention belongs to the technical field of pathogenic microorganism detection, and particularly provides an indirect ELISA system for detecting a clostridium perfringens beta2 toxin antibody, which is good in specificity, high in sensitivity and good in repeatability, and provides an effective detection means for the clostridium perfringens infection condition of animals. The indirect ELISA detection method for the clostridium perfringens beta2 toxin antibody has a wide application prospect in the aspects of clinical serum epidemiological investigation and vaccine immune effect evaluation, and can be used for rapidly detecting the clostridium perfringens beta2 toxin antibody.
Owner:NINGXIA UNIVERSITY
Capture ELISA (enzyme-linked immunosorbent assay) detection method for Clostridium perfringens Alpha-toxin antibody
InactiveCN106896228AImprove accuracyImprove featuresMaterial analysisSerodiagnosesClostridium perfringens toxoid
The invention relates to the field of animal bacteriology and provides a capture ELISA (enzyme-linked immunosorbent assay) detection method for a Clostridium perfringens Alpha-toxin antibody; in the method, prepared type-A Clostridium perfringens exotoxin is used as an antigen that is used for immunizing a rabbit to obtain rabbit serum and for acting as a binding antigen for capture ELISA after being concentrated and purified; the detection method comprises the steps of (1) enveloping; (2) sealing; (3) adding an antigen; (4) adding a sample to be tested; (5) adding a secondary antibody; (6) developing color; (7) ending; (8) judging results. The detection method has the advantages of high specificity, high sensitivity, good repeatability, low cost and the like; in addition, the detection method is suitable for detecting samples in large batch, and an effective and simple serological diagnostic method is provided for the detection and research on such toxin.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
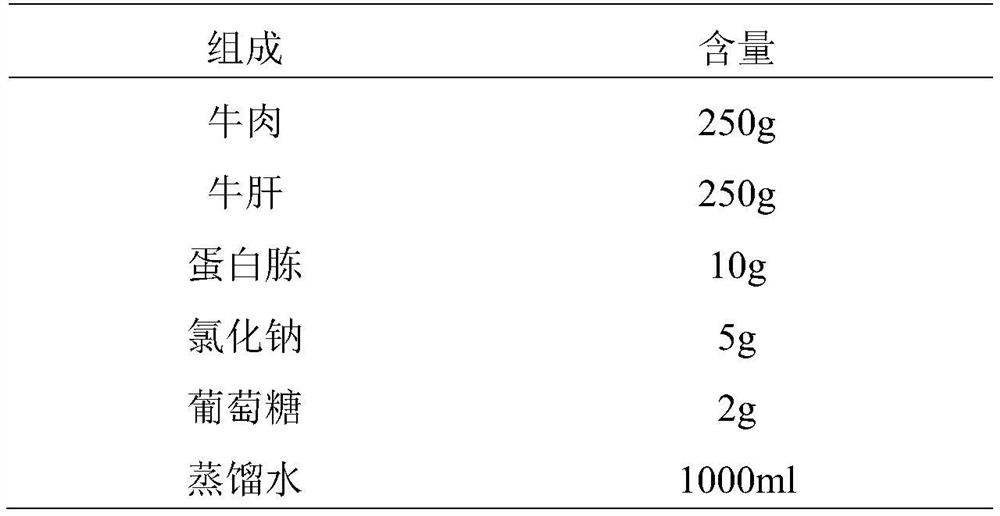
Preparation method and application of veterinary-use A type clostridium perfringens toxins
ActiveCN109943507AThe preparation method is simple and easyLow costAntibacterial agentsBacterial antigen ingredientsClostridium perfringens toxoidBacillus perfringens
The invention discloses a preparation method and application of veterinary-use A type clostridium perfringens toxoids. The preparation method of the toxoids includes the following steps that A type clostridium perfringens production strains are inoculated into a culture medium for fermentation and culture, a fermentation product is obtained, then the fermentation product is inactivated and detoxified in L-lysine and a formaldehyde aqueous solution under the pH of 6.8, and centrifugal supernatant of the successfully inactivated and detoxified qualified bacterial solution refer to toxoids. The culture medium includes casein peptone, yeast extract powder, CaCl2, ZnSO4 7H2O, Na2HPO4 12H2O, KH2PO4, and glucose. The toxicity of the A type clostridium perfringens toxoids prepared by the method can be raised to be 12.5 times of the standard for seedling cultivation, the neutralization titer, in first and second immune serum of domestic rabbits, of the toxoids can be raised to 4 and 50 times ofa traditional process respectively and maximally, and the ratio of the serum titer to cost can be raised to 48.8 and 1216.4 times of the traditional process.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Clostridium perfringens epsilon toxin attenuated mutant and its application
ActiveCN104560780BAchieve complete attenuationImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsTyrosineBacillus perfringens
The invention discloses a toxin attenuation mutant for epsilon toxin of clostridium perfringens and an application of the toxin attenuation mutant for the epsilon toxin of the clostridium perfringens. An epsilon toxin mutant for attenuating toxin in cells or animals is obtained by mutating tyrosine at a site 71 of mature toxin of the epsilon toxin of wide clostridium perfringens into non-aromatic amino acids. The invention further discloses a recombinant expression vector and a recombinant host cell which contain coding genes of the toxin attenuation mutant for the epsilon toxin of the clostridium perfringens. According to the recombined and expressed toxin attenuation mutant for the epsilon toxin of the clostridium perfringens, the complete toxin attenuation of mice in vitro and in vivo can be achieved, and good immunogenicity and immunizing protection are presented in a mouse model. The toxin attenuation mutant for the epsilon toxin of the clostridium perfringens and the coding genes of the toxin attenuation mutant can be used for preparing subunit vaccines of the epsilon toxin of the clostridium perfringens or subunit vaccines of multivalent clostridium toxin.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Poultry vaccine for clostridium perfringens
ActiveUS20210236617A1Antibacterial agentsBacterial antigen ingredientsAlpha-toxinChitosan nanoparticles
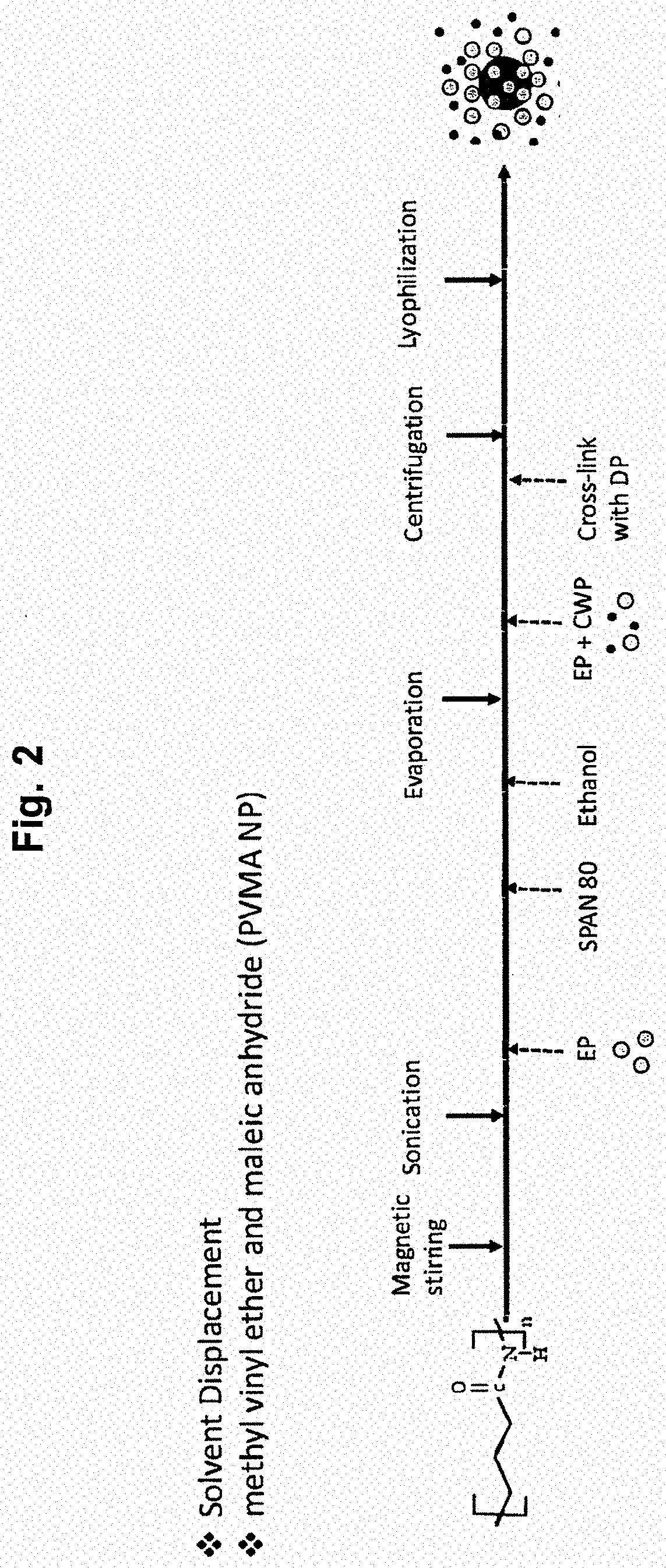
The present disclosure relates to nanoparticle compositions for use as vaccines against Clostridium perfringens in poultry which causes necrotic enteritis in poultry. Such compositions include one or more Clostridium perfringens extracellular proteins entrapped in a polyanhydride or chitosan nanoparticle. The one or more Clostridium perfringens extracellular proteins may include one or more Clostridium perfringens toxins, such as, for example, alpha toxin (CPA), beta toxin (CPB), epsilon toxin (ETX), iota toxin (ITX), perfringolysin O (PFO), enterotoxin (CPE), beta2 toxin (CPB2), or NetB toxin. In some aspects, the composition further includes a Salmonella enteritidis flagellar protein. The present invention also includes methods for the oral delivery of one or more Clostridium perfringens extracellular proteins to the mucosal membrane of the intestinal tract of a bird of the order Galliformes.
Owner:UNIV OF GEORGIA RES FOUND INC +1
Clostridium perfringens alpha toxin double-antibody sandwich ELIS quantitative determination method
ActiveCN103941004BStrong specificityStrong responsivenessMaterial analysisAlpha-toxinClostridium perfringens toxoid
The invention relates to a clostridium perfringens alpha toxin double-antibody sandwich ELIS quantitative determination method. According to the clostridium perfringens alpha toxin double-antibody sandwich ELIS quantitative determination method, alpha toxin protein obtained via prokaryotic expression is taken as an immunogen; monoclonal antibodies obtained via hybridoma technique are taken as detection antibodies and capture antibodies; reaction conditions are optimized via experiments; alpha toxin protein samples with a series of concentration are used for construction of a standard curve; the double-antibody sandwich ELIS method is established; and indexes of the double-antibody sandwich ELIS are verified. The clostridium perfringens alpha toxin double-antibody sandwich ELIS quantitative determination method specifically comprises following steps: (1) prokaryotic expression of alpha toxin; (2) preparation of anti-alpha toxin monoclonal antibodies; (3) establishment of the double-antibody sandwich ELIS method; (4) establishment of the standard curve; and (5) performance evaluation on the double-antibody sandwich ELIS method. The clostridium perfringens alpha toxin double-antibody sandwich ELIS quantitative determination method is excellent in specificity, and high in sensitivity and stability, is fast and convenient, and can be used for effective quantitative determination of alpha toxin in A-E type clostridium perfringens cultural supernatants; and the high-efficient detection method is provided for alpha toxin determination.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Clostridium perfringens beta toxin mutant protein as well as preparation method, application and vaccine thereof
ActiveCN111944028ANon-toxicSuccessfully attenuatedAntibacterial agentsBacterial antigen ingredientsMutated proteinClostridium perfringens beta toxin
The invention provides clostridium perfringens beta toxin mutant protein as well as a preparation method, application and a vaccine thereof, and relates to the field of biological products. The clostridium perfringens beta toxin mutant protein is prepared by mutating 212th arginine and 266th tyrosine of wild clostridium perfringens beta toxin with an amino acid sequence as shown in SEQ ID NO.3 into alanine, and the toxicity of the clostridium perfringens beta toxin mutant protein is remarkably reduced compared with that of the wild clostridium perfringens beta toxin. Meanwhile, good immunogenicity is also reserved.
Owner:天康制药股份有限公司
Clostridium perfringens epsilon toxin mutant protein as well as preparation method, application and vaccine thereof
ActiveCN111961121ASuccessfully attenuatedImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsMutated proteinClostridium perfringens toxoid
The invention provides a clostridium perfringens epsilon toxin mutant protein as well as a preparation method, application and a vaccine thereof, and relates to the technical field of biology. The clostridium perfringens epsilon toxin mutant protein includes alanine mutated by 151-position amino acid of clostridium perfringens epsilon toxin shown in an amino acid sequence SEQ ID NO.3. Compared with a wild type, the clostridium perfringens epsilon toxin protein has significantly reduced toxicity and good immunogenicity. The vaccine can alleviate technical problems such as complex production process, high cost, complex antigen, inter-batch difference and large side effects of inactivated vaccine.
Owner:天康制药股份有限公司
Clostridium perfringens type d toxin for veterinary use and its preparation method and special medium
ActiveCN107299070BGood repeatabilityStrong toxin production abilityAntibacterial agentsBacterial antigen ingredientsClostridium perfringens toxoidBacillus perfringens
The invention discloses a preparation method and a special culture medium of veterinary D-type clostridium perfringen toxin. The culture medium per 100ml comprises the following components: 1-1.5g soy peptone, 1-1.5g casein peptone, 0.5-0.75g yeast extract, 0.5-0.75g Na2HPO4.12H2O, 1-1.5g dextrin and the balance of water, wherein a pH (potential of hydrogen) value of the culture medium is 8.0-8.5. The preparation method of the D-type clostridium perfringen toxin comprises the steps of inoculating a D-type clostridium perfringen production strain into the culture medium, collecting a culture material, performing centrifugation, and then filtering a supernate. With the adoption of the method, the maximum toxicity can be improved to be 45 times a seedling standard in Regulations on National Veterinary Biological Products, and an output-input ratio can be increased to be 30-225 times that of the original traditional technology. In addition, corresponding serum neutralization titer of a toxoid vaccine prepared by the D-type clostridium perfringen toxin on a rabbit and a sheep is also improved to be 8.3 and 13.3 times a regulation standard respectively.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
A Recombinant Fusion Protein of Avirulent Tetanus Toxin and Clostridium perfringens β Toxin
ActiveCN109942718BImprove stabilityImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsVaccine PotencyClostridium perfringens beta toxin
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Type b Clostridium perfringens exotoxin and its preparation method, toxin-producing medium and application
ActiveCN109554420BOptimizing Toxigenic Culture ConditionsStrong toxin production abilityAntibacterial agentsBacterial antigen ingredientsBiotechnologyClostridium perfringens toxoid
The invention discloses a type B Clostridium perfringens exotoxin, a preparation method thereof, a toxin-producing medium and an application thereof. Wherein every 1000ml described toxin-producing medium is made of the raw materials of following weight ratio: peptone 15-25g, yeast extract powder 3-5g, NaCl 3-4g, NaCl 2 HPO 4 12H 2 O 5‑10g, dextrin 5‑10g or crude dextrin 5‑10g, and the rest is water for injection. The virulence of the type B Clostridium perfringens toxin prepared by the method of the invention can reach up to 1 mouse MLD≤0.0006mL, which is 3.3 times higher than the seedling production standard. Moreover, the neutralization titer of the corresponding serum neutralization titers of rabbits and sheep in the triple inactivated vaccine of sheep rapid epidemic disease and sudden attack (or lamb dysentery) enterotoxemia also reaches or exceeds the standard of relevant regulations.
Owner:JINYUBAOLING BIO PHARMA CO LTD
Clostridium perfringens alpha toxin humanized single chain antibody 7d
ActiveCN104531731BSmall molecular weightStrong penetrating ability in vivoAntinoxious agentsAntibody ingredientsPhage antibodiesAlpha-toxin
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Clostridium perfringens alpha toxin visual detection method
PendingCN111983240AImprove stabilityHigh sensitivitySerum immunoglobulinsImmunoglobulins against bacteriaBiotechnologyMicrosphere
The invention discloses a Clostridium perfringens alpha toxin visual detection method. A smartphone image processing technology and silicon dioxide microsphere antibody coupling are combined to detectfood-borne clostridium perfringens alpha toxin (CPA). According to a double-antibody sandwich principle, the prepared CPA polyclonal antibody is coupled with silicon dioxide microspheres, CPA fluorescence detection is performed through antibody-labeled FITC fluorescence, and finally, the CPA content is detected in real time in combination with an image processing technology of a smartphone APP. Compared with a traditional clostridial toxin detection method, the nano-microsphere combined intelligent mobile phone CPA detection system is a more sensitive, stable and convenient detection method,and a new method is provided for detection of natural CPA in food.
Owner:TIANJIN UNIV
A kind of preparation method of e-type Clostridium perfringens toxoid vaccine
ActiveCN107875377BImprove immunityNo side effectsAntibacterial agentsBacterial antigen ingredientsBiotechnologyDisease
The invention discloses a preparation method of an E-type clostridium perfringens toxin vaccine. The method comprises the following steps: 1) an E-type clostridium perfringens bacterial strain is inoculated to an aseptic blood dish medium, anaerobic cultivation is carried out, and recovery of the bacterial strain is performed; 2) the recovered E-type clostridium perfringens bacterial strain is inoculated to a liquid thioglycollate fluid medium, enrichment culture is carried out under anaerobic condition, and seed liquid is prepared; 3) the seed liquid is inoculated to a toxin-producing medium,anaerobic cultivation is carried out, and exotoxin liquid is prepared; and 4) the prepared exotoxin liquid is subjected to inactivation and emulsification, and merthiolate is added to obtain the E-type clostridium perfringens toxin vaccine. The E-type clostridium perfringens toxin vaccine has the advantages of strong immunity, and no toxic and side effect. The vaccine can effectively prevent diseases such as calf and lamb dysentery due to the strain.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Polynucleotides encoding Clostridium perfringens alpha toxin proteins
This invention pertains in part to the development of a vaccine for poultry against necrotic enteritis (NE). The vaccine utilizes a protective antigen that is a mutated, full-length, non-toxic Clostridium perfringens (Cp) α-toxin protein (Mcpa). Utility of this vaccine was demonstrated by reduction of lesion severity in NE challenge trails, for example. Also disclosed herein are novel approaches for producing this vaccine in significant quantities. One exemplified approach involves producing NE vaccine (mutated alpha toxin) in bacterial expression systems, preferably utilizing the Pseudomonas fluorescens system, for commercial use in controlling NE in the poultry industry. The subject vaccines can be administered preferably to chickens in several different ways. A novel, Type VI alpha toxin from chicken isolates of Cp is also disclosed.
Owner:DOW AGROSCIENCES LLC
Clostridium perfringens epsilon toxin recombinant subunit vaccine and production method thereof
ActiveCN107753940BReduce Biosecurity RisksAvoid time costAntibacterial agentsBacterial antigen ingredientsVaccine PotencyVaccine Production
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Clostridium perfringens alpha toxin humanized single chain antibody 8b
ActiveCN104531730BSmall molecular weightStrong penetrating ability in vivoAntinoxious agentsAntibody ingredientsSingle-Chain AntibodiesDamages tissue
The invention discloses a humanized single-chain antibody 8B of Clostridium perfringens α toxin, which is screened from the phage antibody library Source bioscience, and is a fully human anti-Clostridium perfringens α toxin antibody, which can be realized Neutralizing the therapeutic effect of toxins, while overcoming various side effects of heterologous antibodies, eliminating the cumbersome steps and high cost of humanized transformation of heterologous antibodies, due to the small molecular weight of single-chain antibodies and strong penetrating ability in vivo, It quickly reaches damaged tissues and cells to play an anti-toxin effect, thus achieving the dual purpose of economy and high efficiency. The α-toxin has certain homology with other types of toxins of Clostridium perfringens, and the antibody drug may have inhibitory and therapeutic effects on other types of toxins, and can also be used as a detection reagent to detect α-toxins of Clostridium perfringens.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com