Bovine type-A clostridium perfringens subunit vaccine and preparation method and application thereof
A technology for Clostridium perfringens and subunit vaccines is applied in chemical instruments and methods, antibacterial drugs, bacterial antigen components, etc., to achieve the effects of simple process, cheap consumables, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
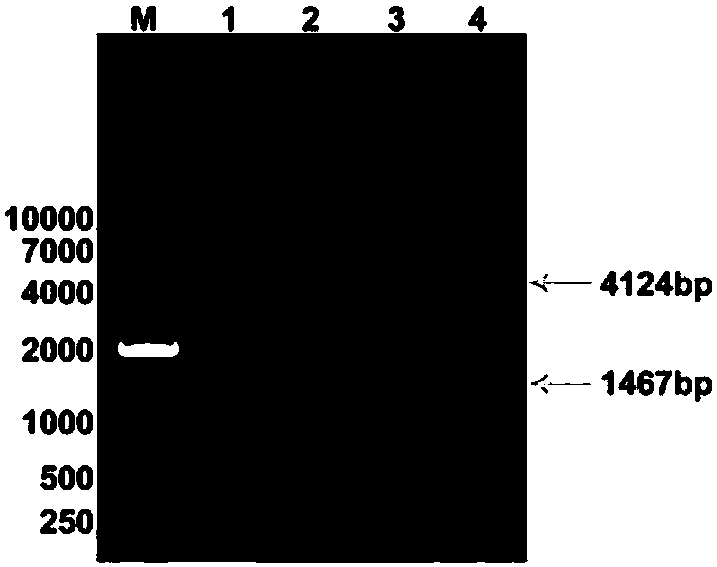
Image
Examples
Embodiment 1
[0022] The preparation of embodiment 1 alpha-toxin protein truncated body
[0023] 1. Selection of truncation position of 1α-toxin protein truncated body
[0024] Alpha toxin is the main pathogenic factor of Clostridium perfringens type A, which can stimulate the body to produce neutralizing antibodies. It itself is a metalloenzyme dependent on the activity of zinc ion phospholipase C (PLC), which can hydrolyze the phosphatidylcholine in the cell membrane, destroy the integrity of the cell membrane, and eventually lead to cell rupture and death. The structure of α-toxin protein (PDB: 1GYG) was analyzed, and its structure can be divided into two domains: the N-terminal structure includes nine tightly connected α-helices, and the C-terminal structure consists of 8 antiparallel β-sheets , N-terminal and C-terminal are connected by a short and flexible linker. The N-terminal domain is completely present in the phospholipase active region, while the C-terminal region containing a...
Embodiment 2
[0118] Example 2 α-toxin protein truncated mouse toxicity test
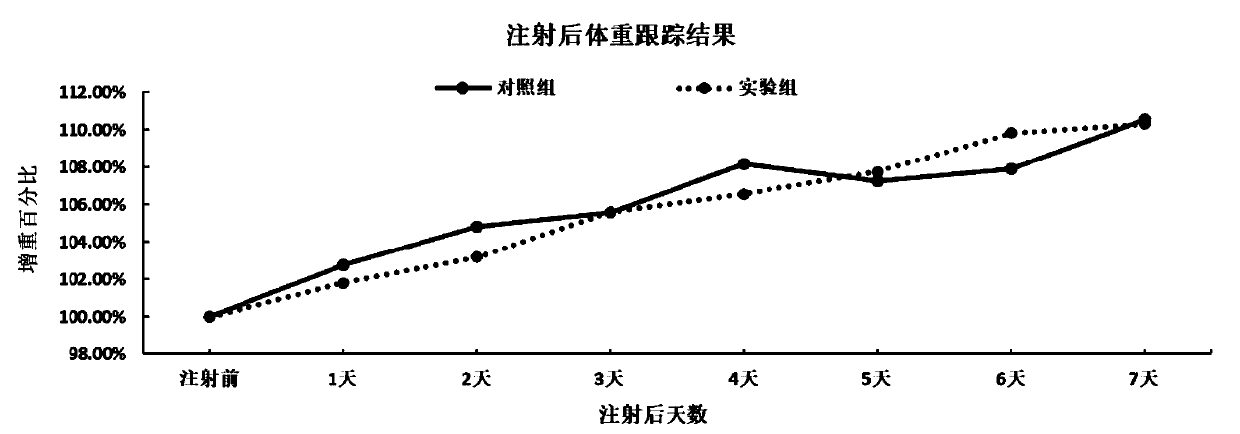
[0119] Purchase 10 18-20g BALB / C mice and randomly divide them into 2 groups with 5 mice in each group. One group was used as the control group, and 200 μl of PBS was injected into the leg muscles; the other group was injected with 200 μl (the total amount of protein was 200 μg) of α-toxin protein truncated body. Observed continuously for 7 days after injection, and observed mental appetite and measured body weight every day.
[0120] Within 7 days of injection follow-up, the mental appetites of the mice in the two groups were all good, no significant difference was seen, and the weight gain (see image 3 ) and there is no significant difference. This shows that the truncated α-toxin protein prepared by us is safe, and the tolerated amount of the mouse is very high (up to 200 μg at the lowest), which far meets the requirements for vaccine preparation.
Embodiment 3
[0121] Example 3 Preparation of bovine type A Clostridium perfringens subunit vaccine
[0122] Add an appropriate amount of truncated α-toxin protein to ISA 201VG adjuvant (the weight ratio of antigen phase and adjuvant is 1:1, emulsify and store at 4°C after passing the quality inspection; the specific vaccine information is shown in the following table:
[0123]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com