Monoclonal antibody, vaccine composition thereof and application thereof
A monoclonal antibody and vaccine composition technology, applied in the direction of antibodies, antiviral immunoglobulins, antiviral agents, etc., can solve the problems of no effective prevention and control measures, and achieve excellent results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0023] As an embodiment of the present invention, the amino acid sequence of the heavy chain variable region of the antibody is SEQ ID No.2, and the amino acid sequence of the light chain variable region is SEQ ID No.4.
[0024] As an embodiment of the present invention, the antibody is monoclonal antibody 1H1, the amino acid sequence of the heavy chain variable region of the monoclonal antibody 1H1 is SEQ ID No. 2, and the amino acid sequence of the light chain variable region is SEQ ID No. 2. ID No.4.
[0025] As an embodiment of the present invention, the antibody is monoclonal antibody 1H1, and the amino acid sequence of the heavy chain variable region of the monoclonal antibody 1H1 is the nucleotide sequence shown in SEQ ID No.1 or its degenerate sequence The coding, and the amino acid sequence of the light chain variable region are coded by the nucleotide sequence shown in SEQ ID No.3 or its degenerate sequence.
[0026] The monoclonal antibody 1H1 is an anti-pseudorabi...
Embodiment 1
[0056] Embodiment 1 Preparation, Identification and Inspection of Anti-Porcine Pseudorabies Virus Monoclonal Antibody
[0057] 1.1 Preparation of monoclonal antibody against porcine pseudorabies virus
[0058] Porcine pseudorabies virus HN1201 strain (see Chinese patent CN103923884A) was inoculated into BHK21 cells, and the virus was harvested when the cytopathic rate was 85%, frozen and thawed once at -40°C, centrifuged at 3000r / min for 30min, and inactivated after removing cell debris, and then According to Harlow E etc. (Harlow E, Lane D.Antibodies: a laboratory manual.NewYork:Cold Spring Harbor Laboratory Press, 1998,139-312) the operation method of literature preparation mouse hybridoma cell, this mouse hybridoma cell secretes anti- Porcine pseudorabies virus monoclonal antibody 1H1, the monoclonal antibody is prepared by the method of producing monoclonal antibody from mouse ascites.
[0059] 1.2 Identification of monoclonal antibodies against porcine pseudorabies virus...
Embodiment 2
[0088] Example 2 Application of Monoclonal Antibody 1H1 to Prevent and Treat Porcine Pseudorabies
[0089] 2.1 Evaluation of the effect of monoclonal antibody 1H1 on preventing porcine pseudorabies
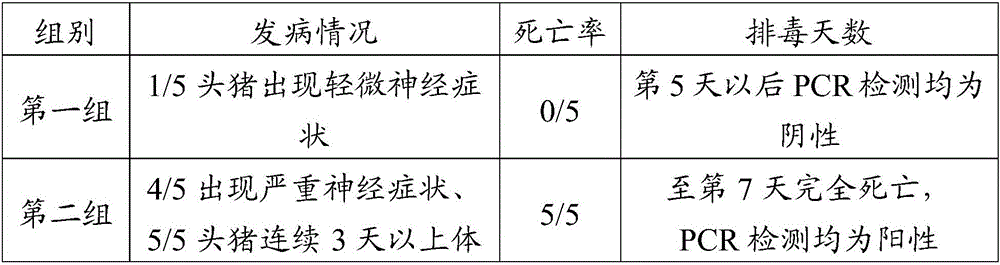
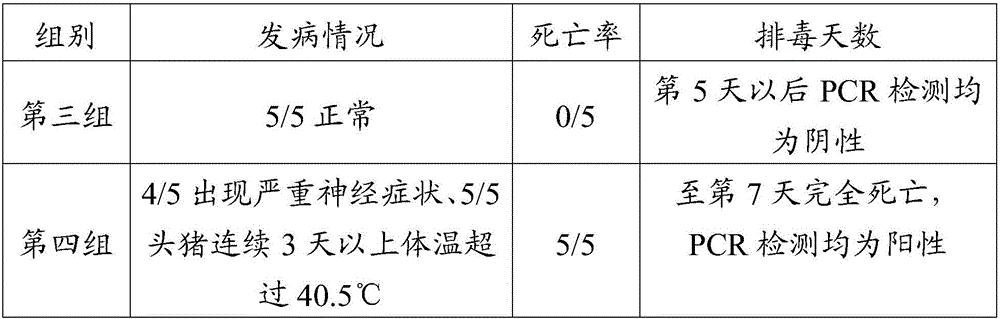
[0090] Screen 10 1-3 day-old piglets of PRV antigen, antibody double negative, be divided into 2 groups at random, the monoclonal antibody 1H1 2ml prepared in the first group intramuscular injection embodiment 1, the second group intramuscular injection PBS damping fluid 2ml, After 24 hours of injection, two groups of animals were simultaneously inoculated with porcine pseudorabies virus HN1201 strain 1ml (10 7.0 TCID 50 / ml), observe the clinical symptoms of piglets every day, observe continuously for 14 days, and evaluate the prevention of piglets by monoclonal antibody 1H1 by the clinical morbidity, morbidity, mortality and the number of days of virus monitoring and detoxification in nasal swabs by PCR method. The effect of pseudorabies, the results are shown in Table 2.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com