Rabies virus resistant specific humanized antibody and application thereof
A rabies virus and antibody technology, applied in antiviral agents, antiviral immunoglobulins, antibodies, etc., can solve the problem of no drugs being approved for marketing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1. Construction and rescue of anti-rabies virus ScFv phage antibody library
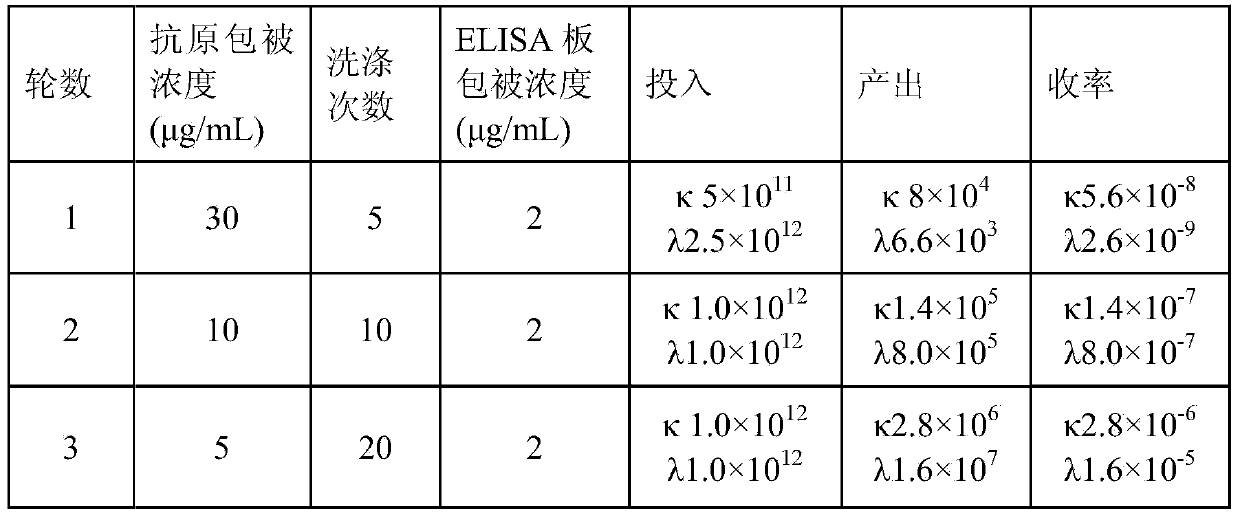
[0040] The anti-rabies virus ScFv phage antibody library is independently constructed by our laboratory. It is based on 32 high-potency healthy human peripheral blood vaccinated against rabies as raw materials. It is extracted from lymphocytes, total RNA is extracted, cDNA is reverse transcribed, and the heavy chain is obtained by PCR. and the light chain variable region gene, and obtained ScFv by overlapping extension PCR (Splicing overlap extension PCR, SOE-PCR). ScFv was digested and ligated with phagemid pS100, and finally electroporated into competent Ecoli TG1 cells to obtain the primary antibody library. After testing, the library capacity of the primary antibody library is about 9.0×10 8 .
[0041] Inoculate the H+λ primary antibody library bacterial solution and the H+κ primary antibody library bacterial solution into 2YT-AG at a ratio of 50 times the capacity of the primary ...
Embodiment 2
[0042] Example 2. Screening of anti-rabies virus phage antibody library
[0043] Prepare inactivated and purified rabies virus using conventional methods. After inactivation and purification, the protein content of rabies virus is 300 μg / ml, which is used for the screening of phage antibody particles.
[0044] Three rounds of selection were performed using purified rabies virus coated immunotubes. The three rounds of antigen coating concentration were 30, 10 and 5 μg / ml in turn, and the washing times were 5, 10 and 20 rounds in turn. The phage particles obtained after screening were re-infected with Ecoli TG1 and spread on 2YT-AG plates, picked clones and cultured in 96-well plates overnight with 2YT-AG, and cultured with 2YT-AK after adding MK1307 helper phage infection. The supernatant was taken and added to an ELISA plate coated with 2 μg / ml purified rabies virus, the color was developed using horseradish peroxidase-labeled mouse anti-M13 antibody, and the absorbance was ...
Embodiment 3
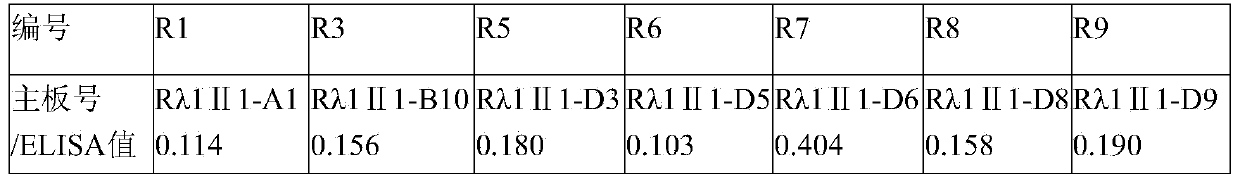
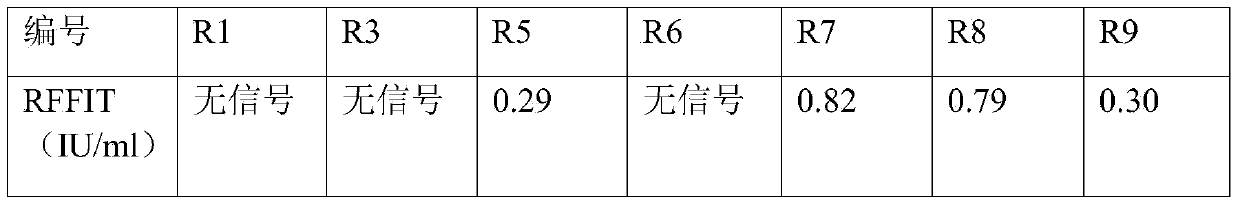
[0048] Example 3, ELISA analysis and screening of candidate antibody monoclonal
[0049] Pick clones from the plate used for titration of phage antibody after enrichment screening and put them in 100 μL of 2×YT-AG. At the same time, pick pS100 empty vector as a negative control, and 3 culture medium only as a blank control, and culture overnight at 37°C. Transfer 10 μL of the culture to a new 96-well plate containing 90 μL of 2×YT-AG, and incubate at 37°C for 1 h. M13K07 was added to each well (to make the phage titer in the culture solution reach 109 cfu / mL, about 25 μL), the MOI was 10-20, and incubated at 37° C. for 30 minutes. Centrifuge at 4000g for 10min, carefully discard the supernatant, resuspend the cells in 100μL of 2×YT-AK, and culture overnight at 30°C.
[0050] The next day, centrifuge at 4000g for 10 min, and use the supernatant for ELISA detection. Coat a 96-well plate with purified rabies virus, 2 μg / mL, 100 μL / well, overnight at 4°C. Wash 3 times with PBST...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com