A kind of monoclonal antibody against novel coronavirus and application thereof
A monoclonal antibody, antibody technology, applied in antiviral agents, antiviral immunoglobulins, antibodies, etc., can solve the problem of no specific drugs for the new coronavirus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
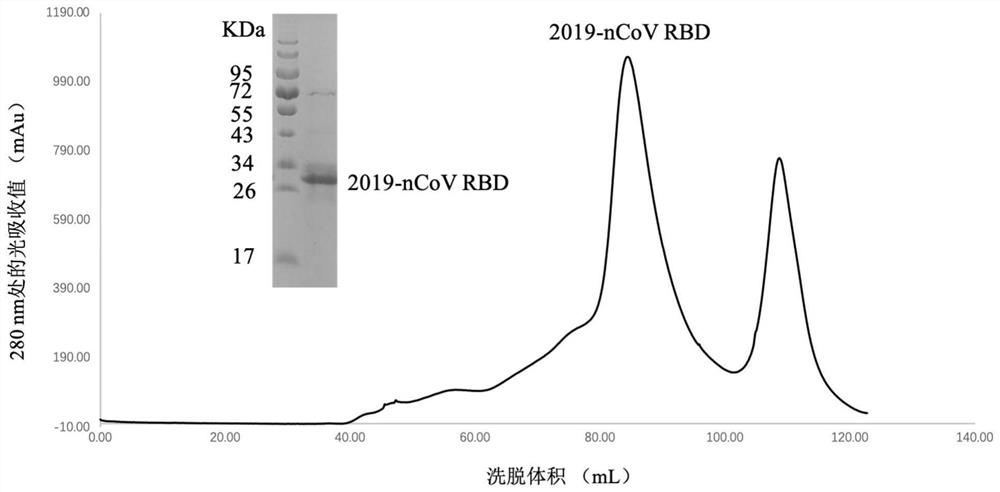
[0093] Example 1: Expression and purification of 2019-nCoV virus S protein RBD
[0094] Using NdeI and XhoI enzymes, the DNA fragment encoding the 2019-nCoV / 2019 strain spike protein S protein RBD (its amino acid sequence is shown in SEQ ID NO: 17) was ligated into the pET21a vector, and the DNA fragment was in the coding region. A nucleotide sequence encoding a 6*histidine tag (6*His tag) and a stop codon are also connected to the 3' end of . The ligation product was transformed into BL21 E. coli competent cells. Then, pick a single clone, inoculate it into 40 mL of LB medium, and cultivate for 6-8 hours; then transfer it to 4 L of LB medium, and cultivate it to OD600=0.4-0.6 at 37 degrees Celsius. Subsequently, IPTG was added to the culture to a final concentration of 1 mM and the incubation was continued for 4-6 hours at 37°C. After the culture, the inclusion bodies were harvested and renatured. The renatured protein solution was concentrated and dialyzed into 20 mM Tris...
Embodiment 2
[0095] Example 2: Isolation of memory B cells that specifically recognize RBD proteins
[0096] With the informed consent of persons infected with 2019-nCoV virus and recovered and discharged, 10 mL of blood was collected to isolate PBMCs. The isolated PBMCs were divided into 10 7 The density / mL was combined with RBD protein (prepared as in Example 1) at a final concentration of 400 nM and incubated on ice for half an hour; then washed twice with PBS and incubated with the following antibodies (both from BD): anti-human CD3 / PE-Cy5, anti-human CD16 / PE-Cy5, anti-human CD235a / PE-Cy5, anti-human CD19 / APC-Cy7, anti-human CD27 / Pacific Blue, anti-human CD38 / APC, anti- human IgG / FITC, and anti-His / PE. After incubation on ice for half an hour, PBMCs were washed twice with PBS. Subsequently, PBMCs were sorted with FACSAria III and PE was collected - Cy5 - APC - APC-Cy7 + Pacific Blue + FITC + PE + cells (i.e., B cells) were collected directly into a 96-well plate, 1 cell / well...
Embodiment 3
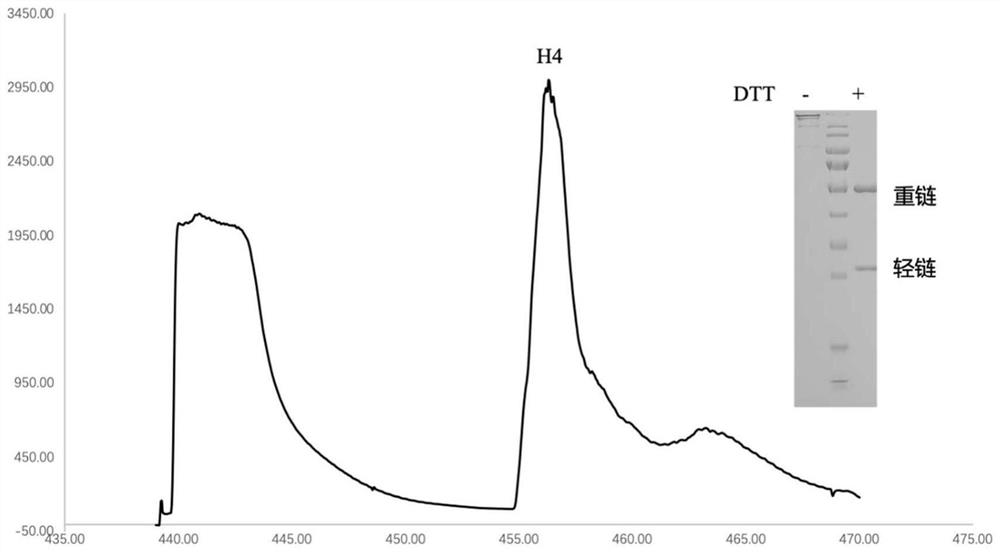
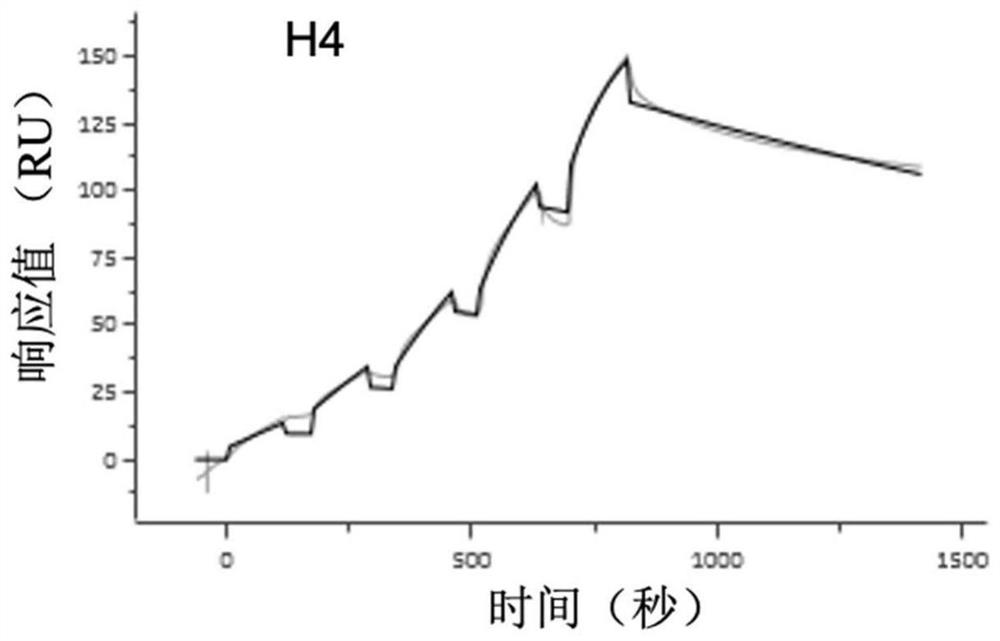
[0097] Example 3: Isolation and identification of H4 antibody and construction of recombinant expression vector
[0098] The B cells obtained in Example 2 were reverse transcribed (at 55°C, for 60 minutes) using Superscript III reverse transcriptase (Invitrogen), wherein the reverse transcription primers used are shown in Table 2.
[0099] Table 2. Sequence information of reverse transcription primers used
[0100]
[0101] Using the reverse transcription product as a template, the first round of PCR (PCRa) was performed with HotStar Tap Plus enzyme (QIAgen) to amplify the sequence of the variable region of the antibody; the primers used were shown in Table 3; the reaction conditions used were As follows: 95°C, 5 min; 35 cycles (95°C 30s, 55°C (heavy chain / kappa chain) 30s, 72°C 90s); 72°C, 7min. Subsequently, a second round of PCR (PCRb) was performed using the amplified product as a template; the primers used were shown in Table 4; the reaction conditions used were as fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com