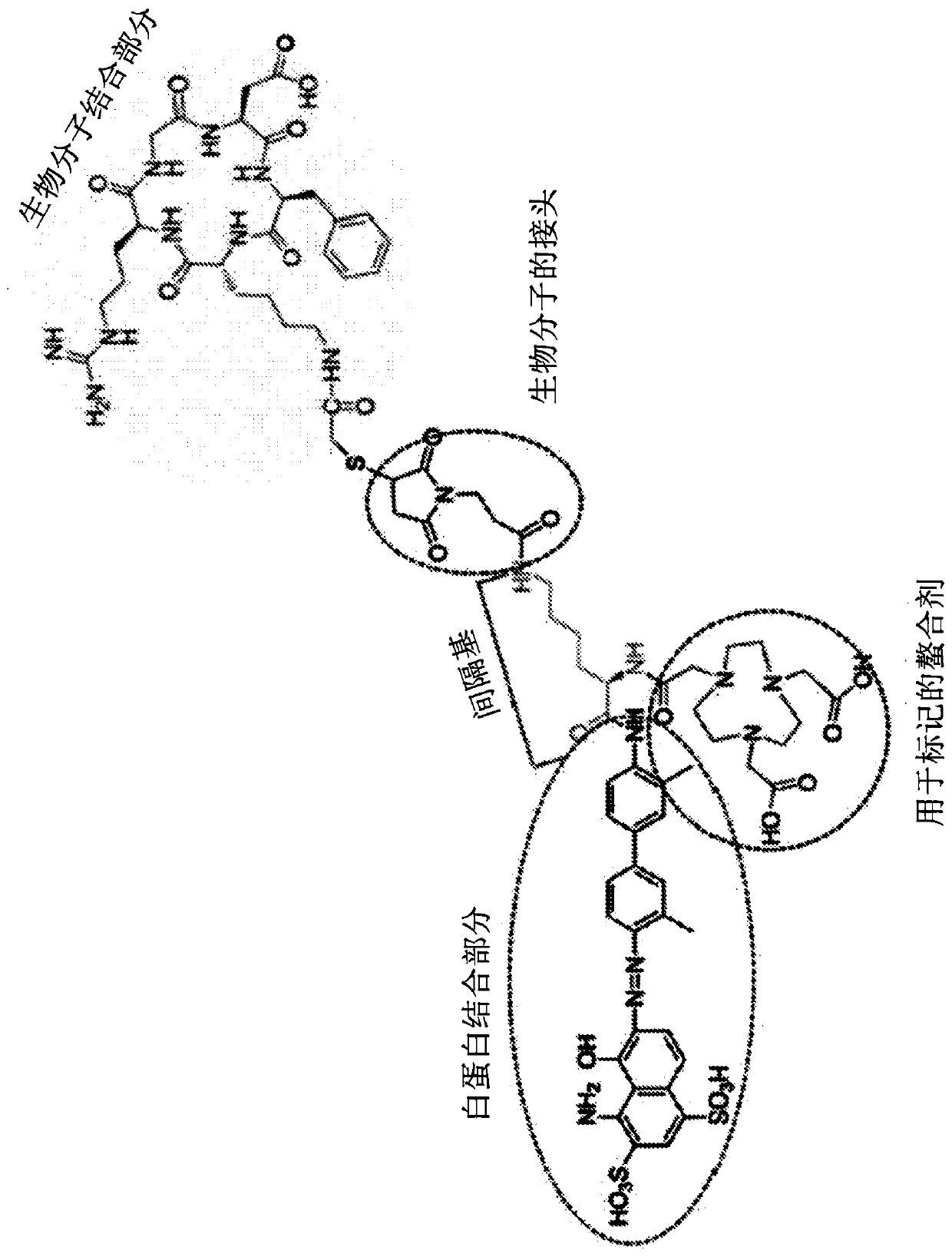
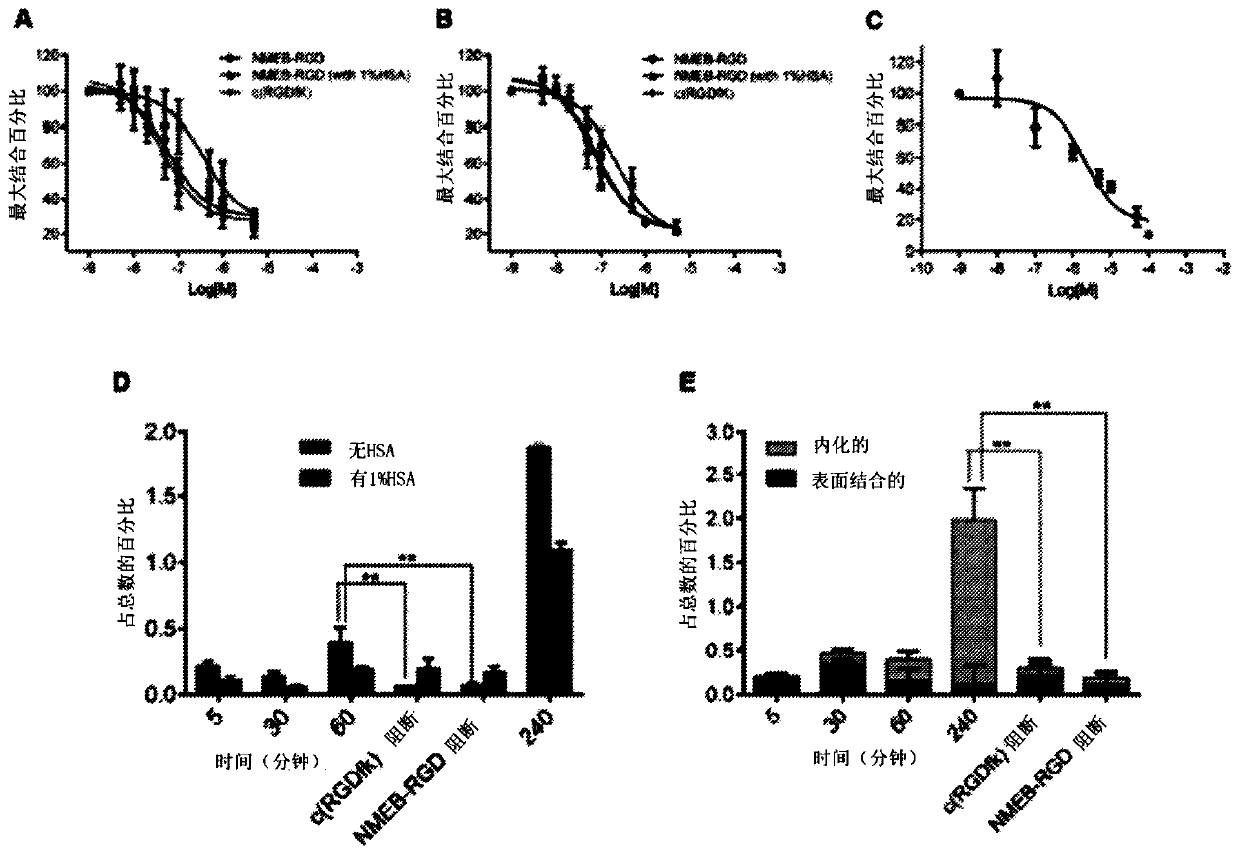
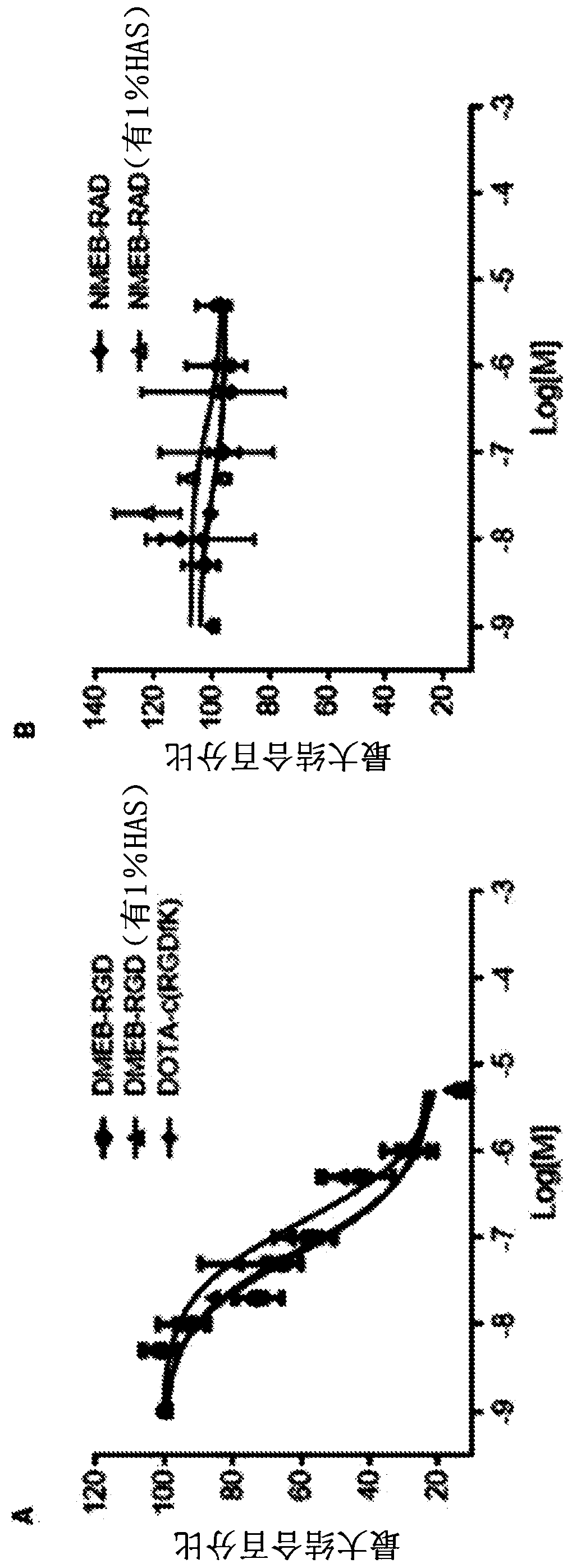
Chemical conjugates of evans blue derivatives and their use as radiotherapy and imaging agents
A compound and pharmaceutical technology, applied in the field of functionalized derivatives of Evans blue dye, can solve problems such as increasing insulin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0256] Embodiment 1: Evans blue amine (EB-NH 2 ) preparation
[0257] To a 100ml round bottom flask containing 2-toluidine (4.3g) and dichloromethane (40ml) was added di-tert-butyl dicarbonate (4.4g). The mixture was stirred overnight at room temperature. The reaction was concentrated and the residue was purified by silica gel chromatography to afford 3.2 g of N-Boc-2-benzylidine. LC-MS: [MH] + = 313.4135 (m / z), Calculated: 312.1838.
[0258]
[0259] N-Boc-2-Tolidine (0.46g, 1.47mmol) was dissolved in acetonitrile (10ml) in a glass vial, cooled to 0°C, then hydrochloric acid (0.3M, 15ml) was added. Cold sodium nitrite solution (0.31 g in 5 ml water) was added dropwise and stirred for 20 min, the solution turned bright yellow. This solution was added dropwise at 0°C to another solution containing 1-amino-8-naphthalene-2,4-disulfonic acid monosodium salt (0.59g) and sodium bicarbonate (0.49g) in water (20ml) in a glass vial. The reaction was deemed complete by LC / MS, ...
Embodiment 2
[0263] Embodiment 2: the synthesis of EB-LYS-BOC
[0264]
[0265] To a solution of Boc-Lys-Fmoc (3.6 equiv) in anhydrous N,N-dimethylformamide (DMF) (2-3 mL) under argon was added (1-[bis(dimethylamino) Methyl]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate) (HATU, 4.2 equivalents). The solution was stirred at room temperature (RT) for 10 minutes. Then 10 equivalents of diisopropylethylamine (DIPEA) was added, followed by EB-NH in 5-7 mL of DMF 2 . The reaction was stirred overnight at room temperature. Monitoring of EB-NH Using Analytical HPLC System 1 2 Conversion to EBs conjugated to protected Fmoc-Lys-Boc. EB-NH 2 The retention time was 7.7 minutes for the conjugated EB-protected Lys and 11 minutes for the conjugated EB-protected Lys. After conversion was complete, 20% piperidine (v / v) was added and the reaction was stirred for an additional 1 hour. DMF was removed with a high vacuum oil pump and the reactants were redissolved in methanol / HO 2 ...
Embodiment 3
[0266] Example 3: Synthesis of NOTA-EB-LYS-BOC
[0267]
[0268] The reaction between EB-Lys-Boc and NOTA-bis(t-Bu ester) was performed similarly to the conditions described above. Analytical HPLC system 2 confirmed >90% purity, r.t 29.3 min, mass 1167 [MH] - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com