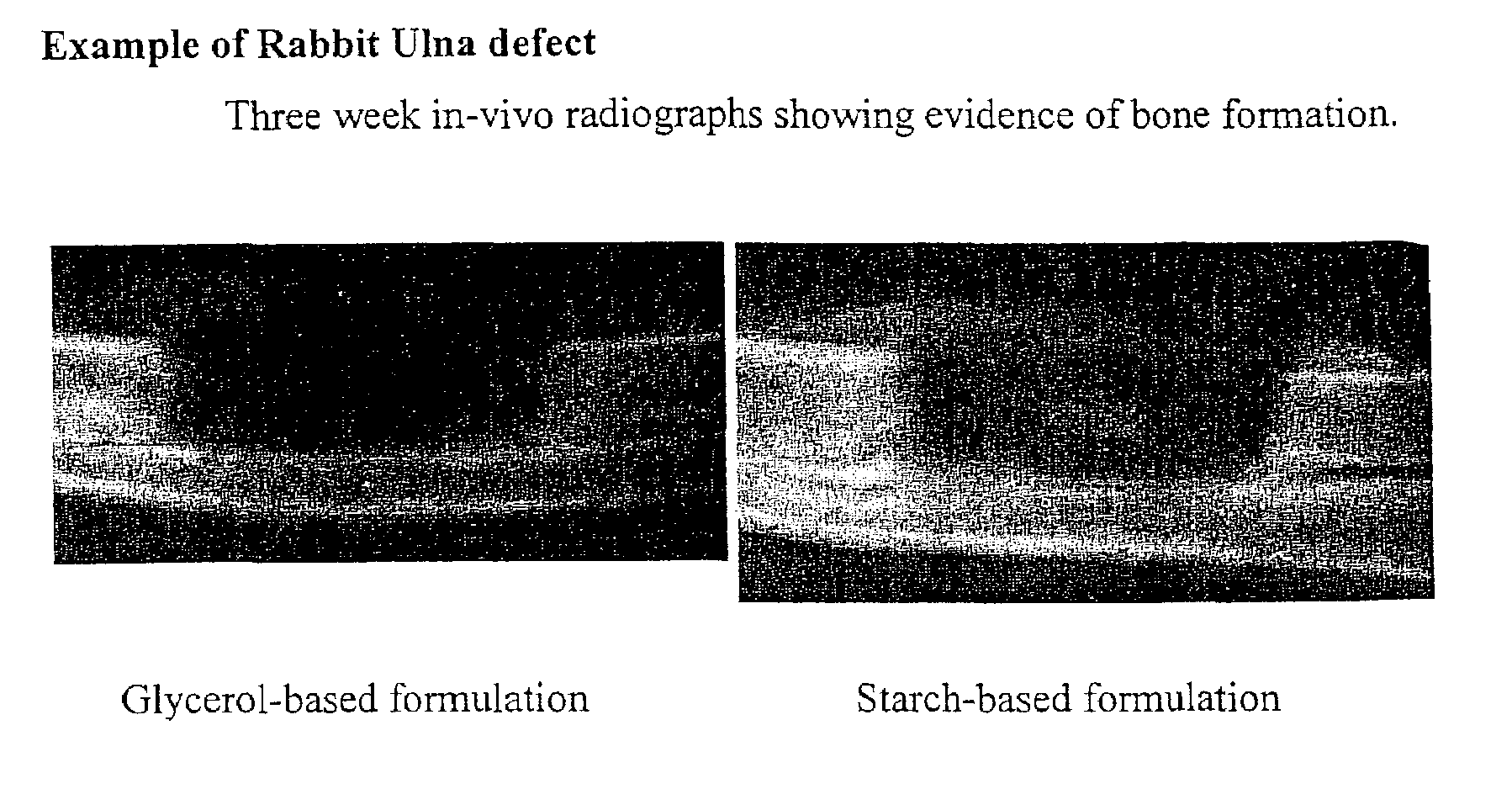
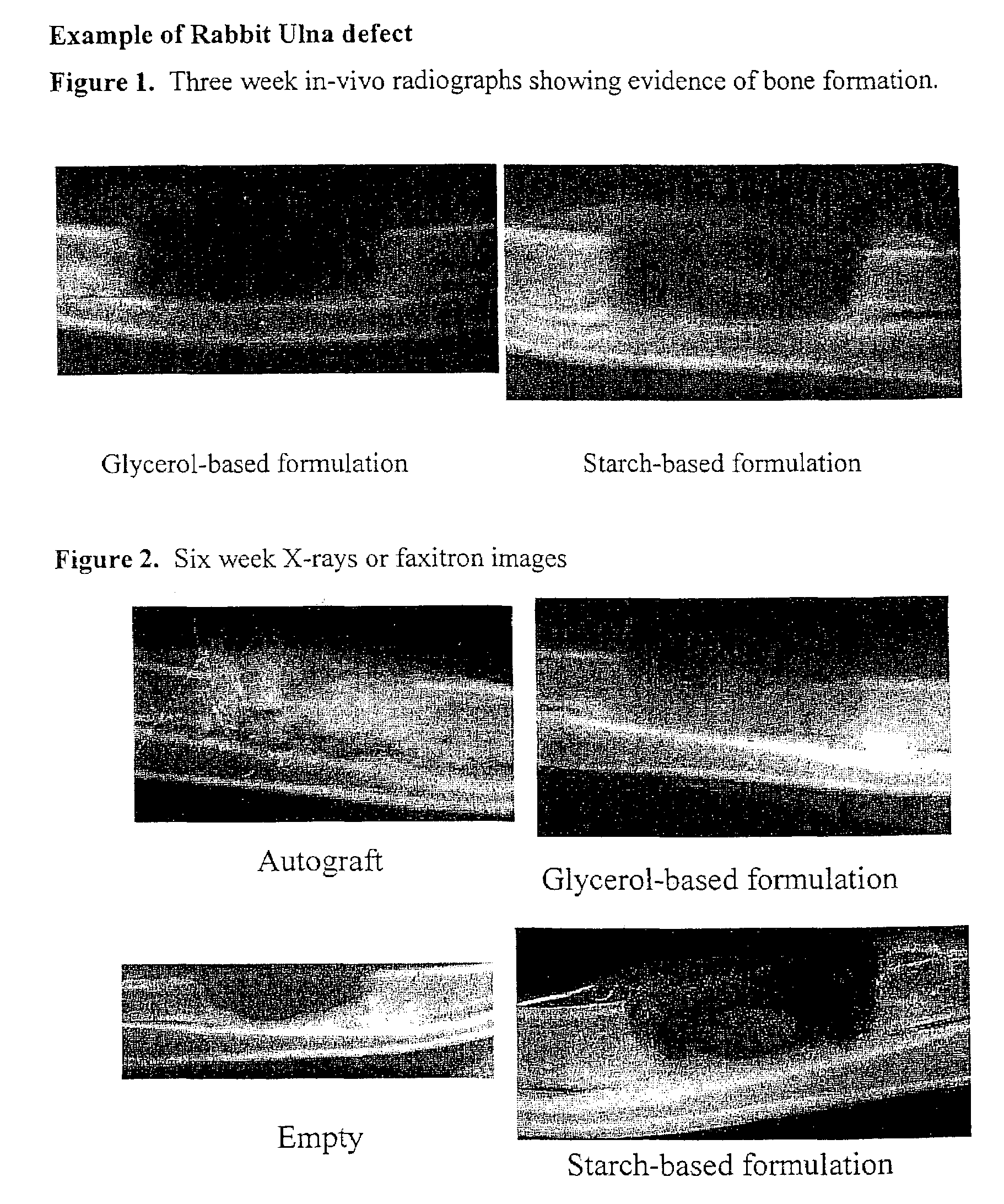
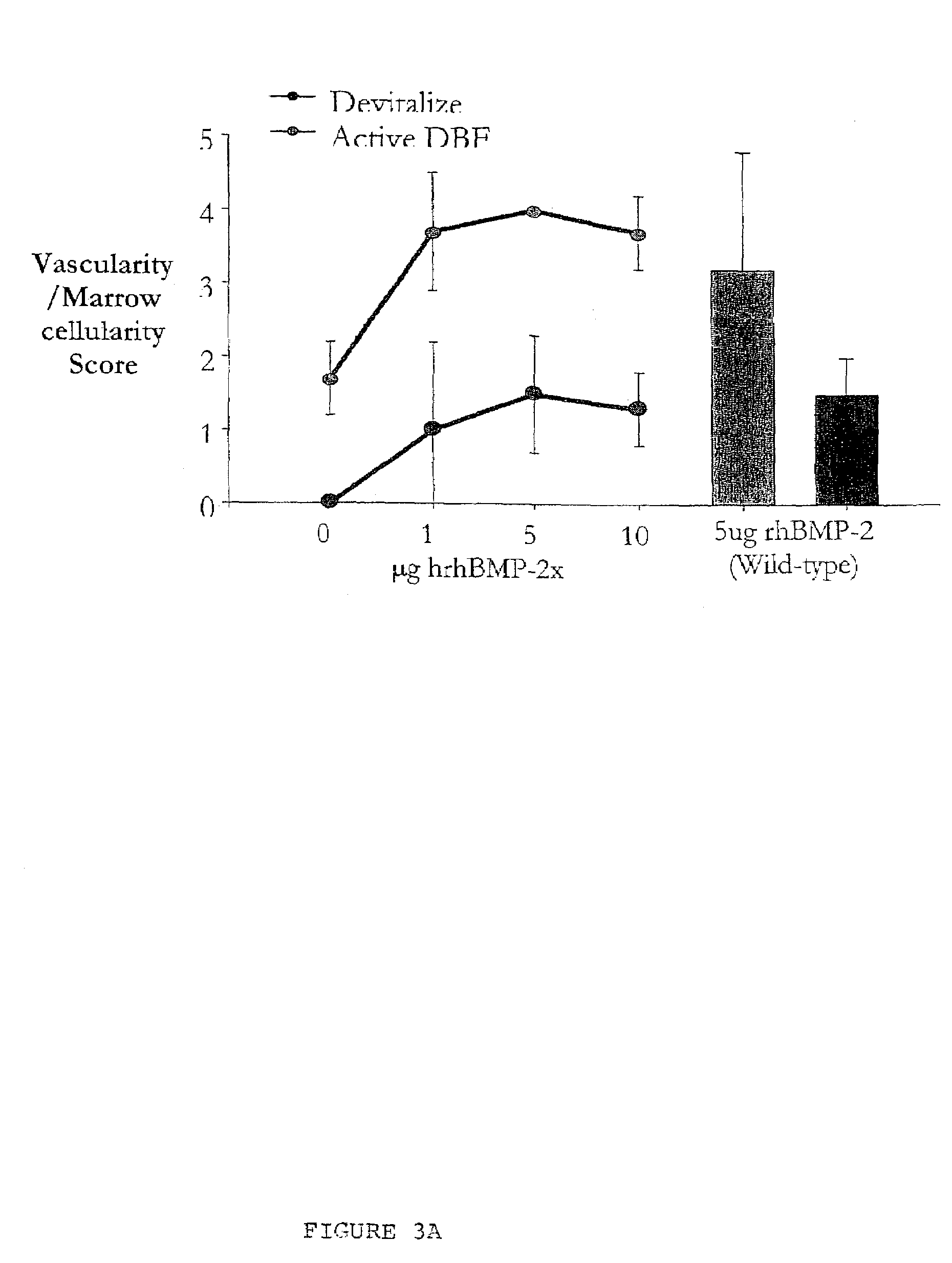
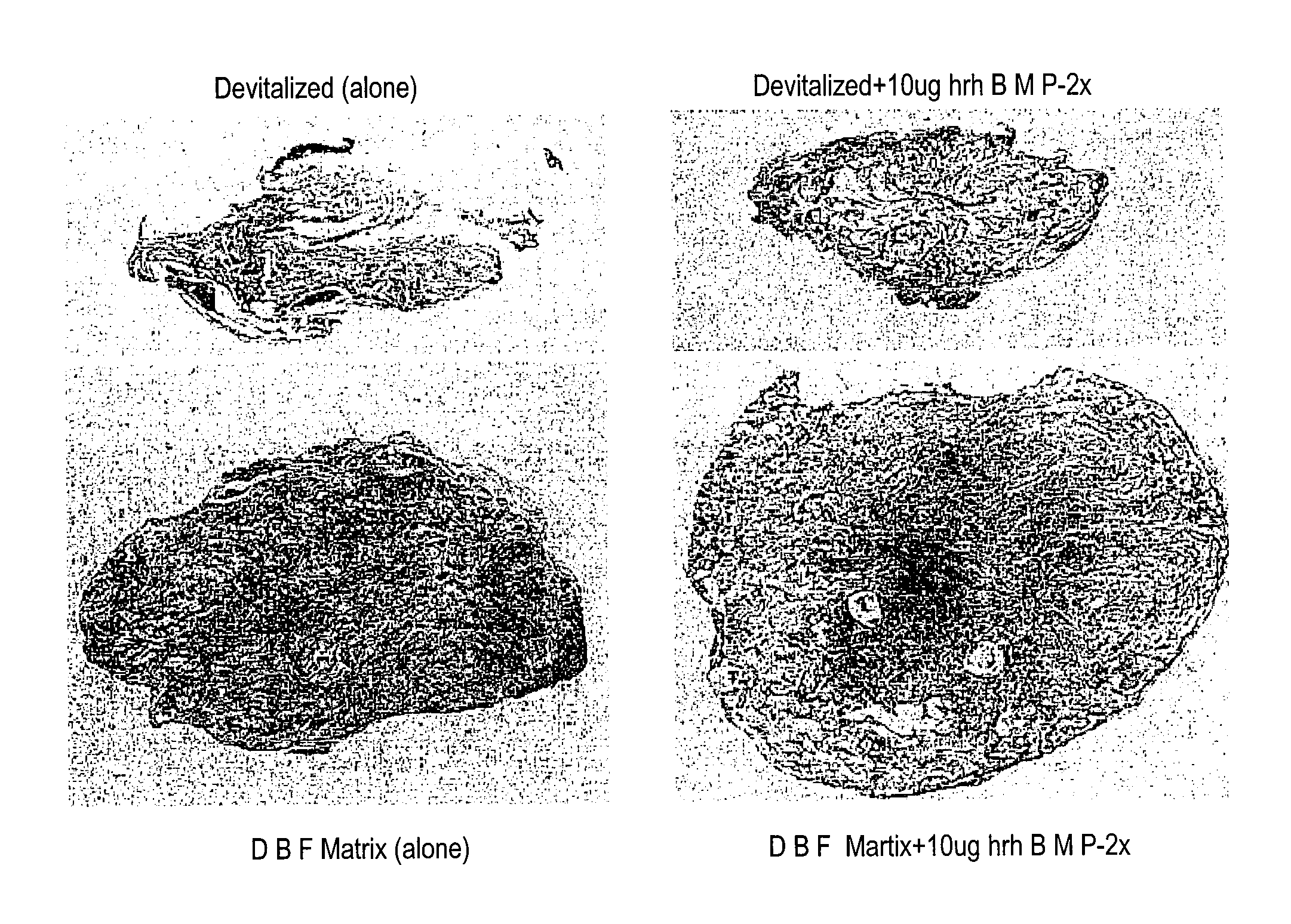
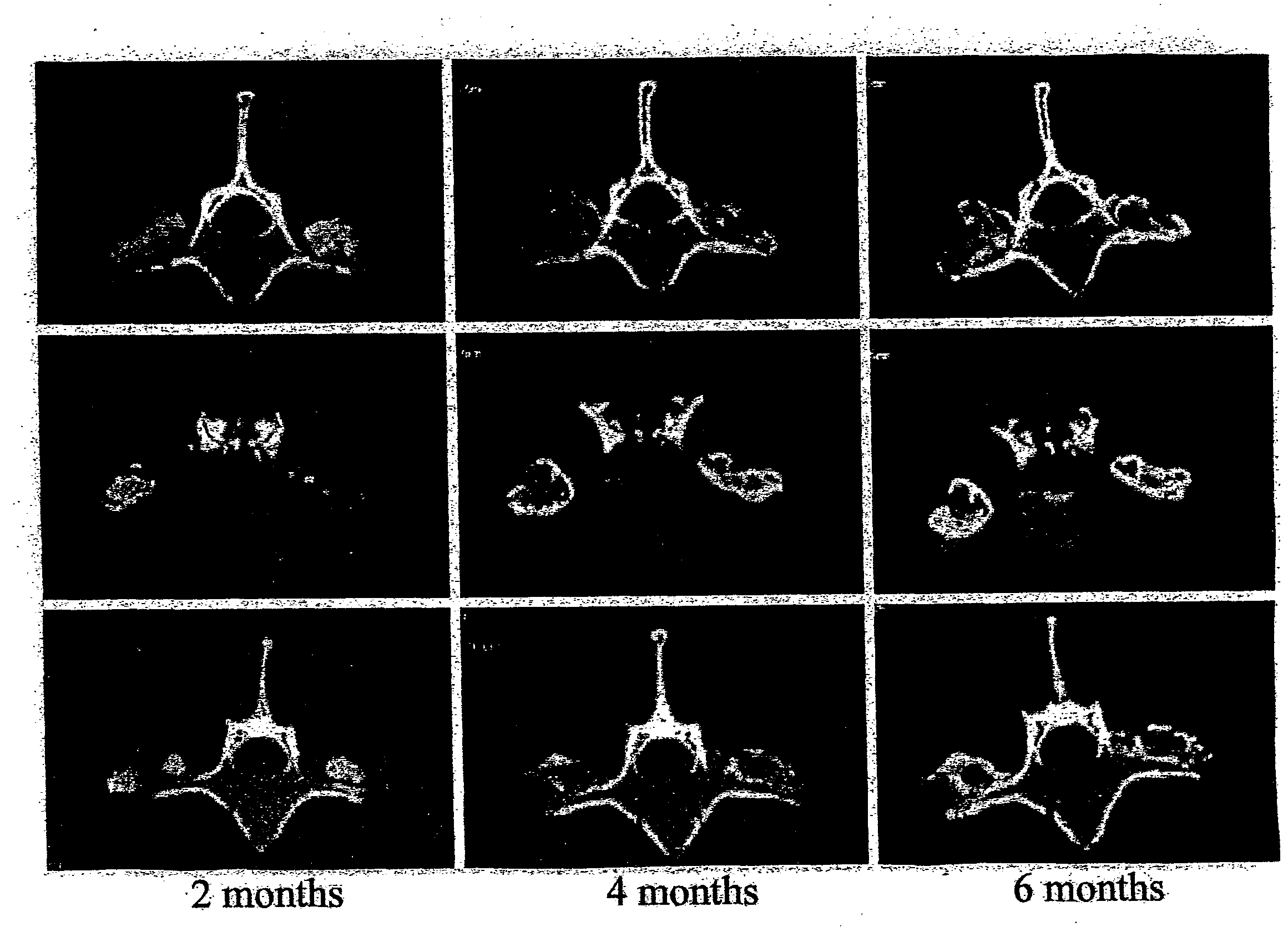
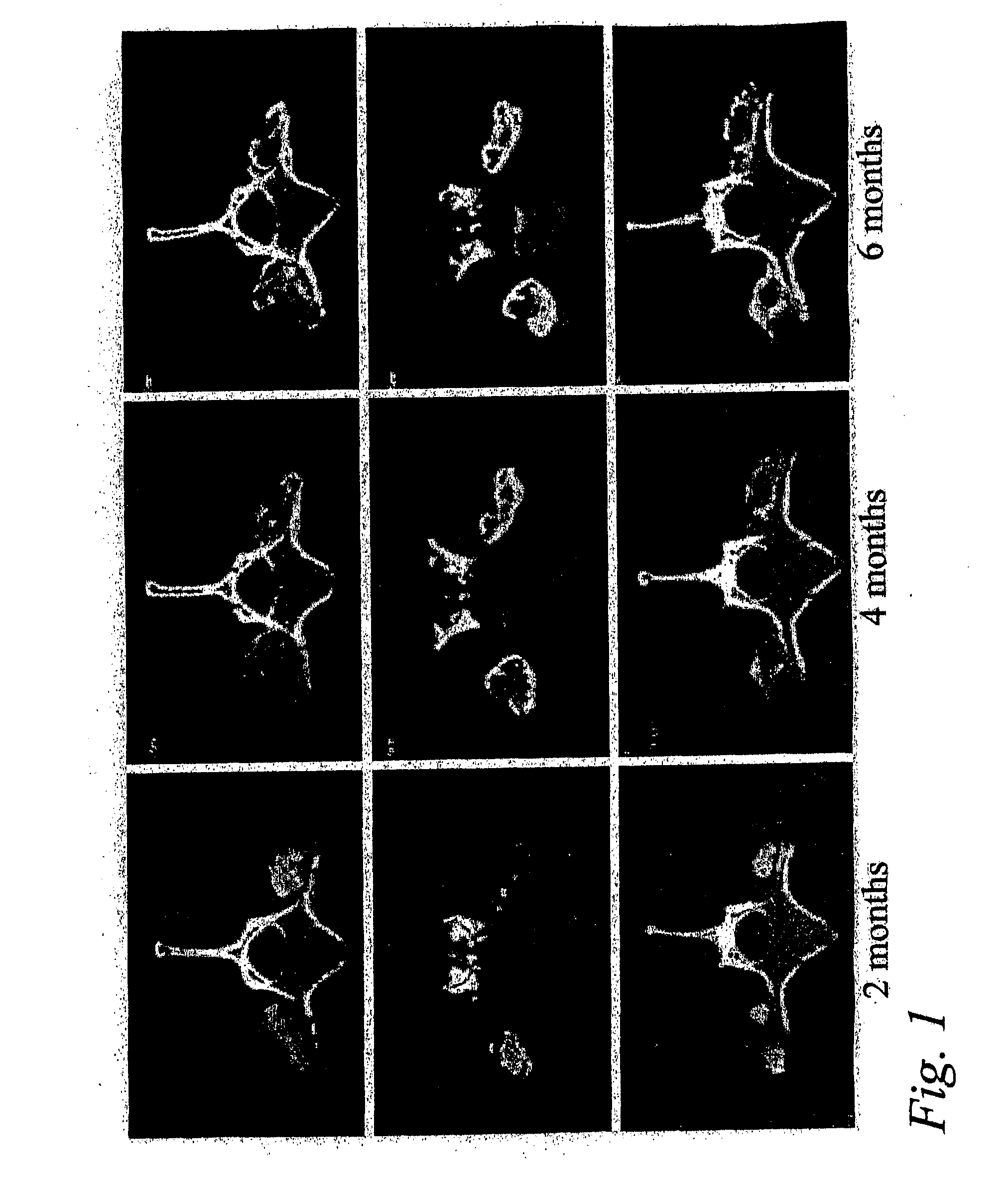
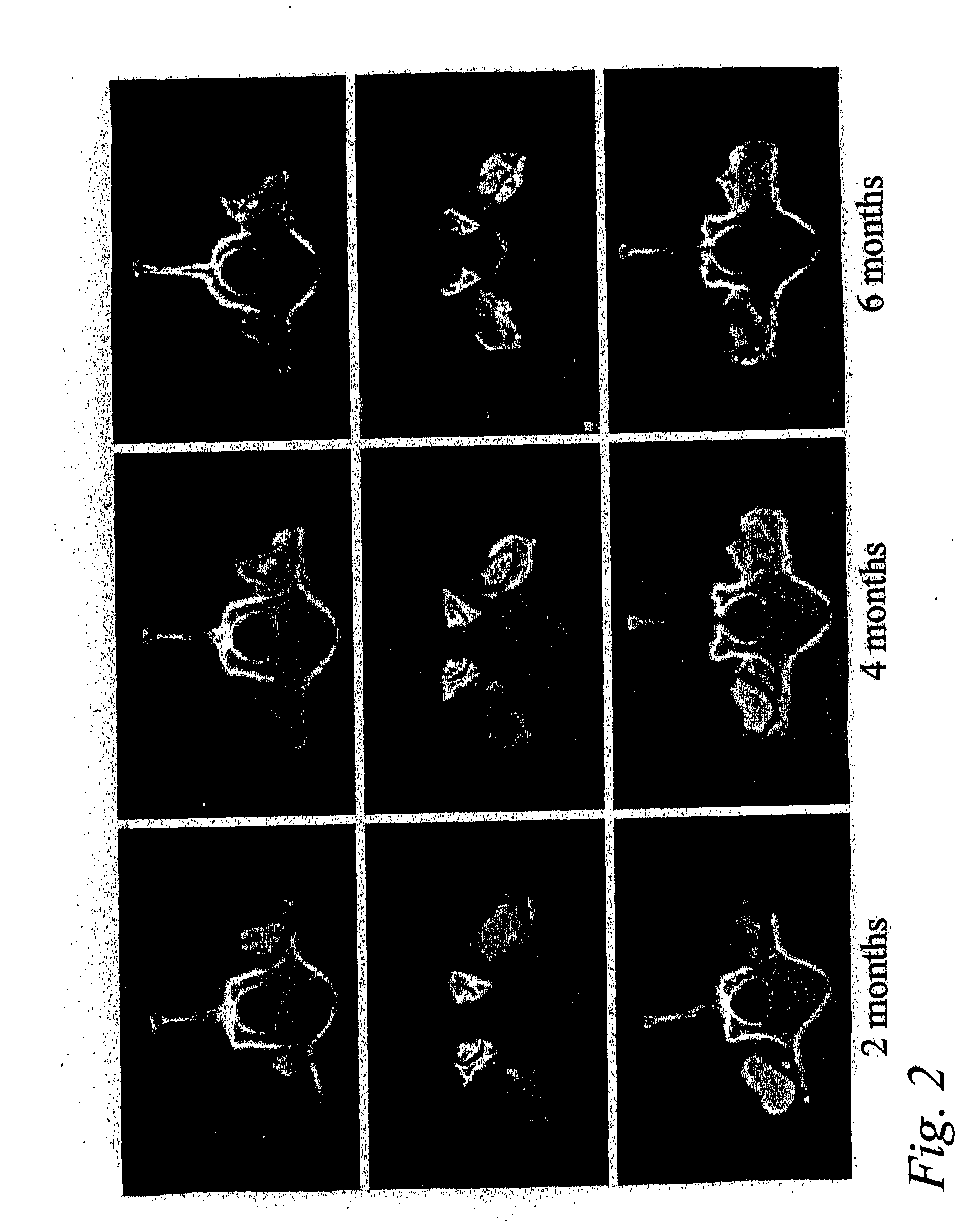
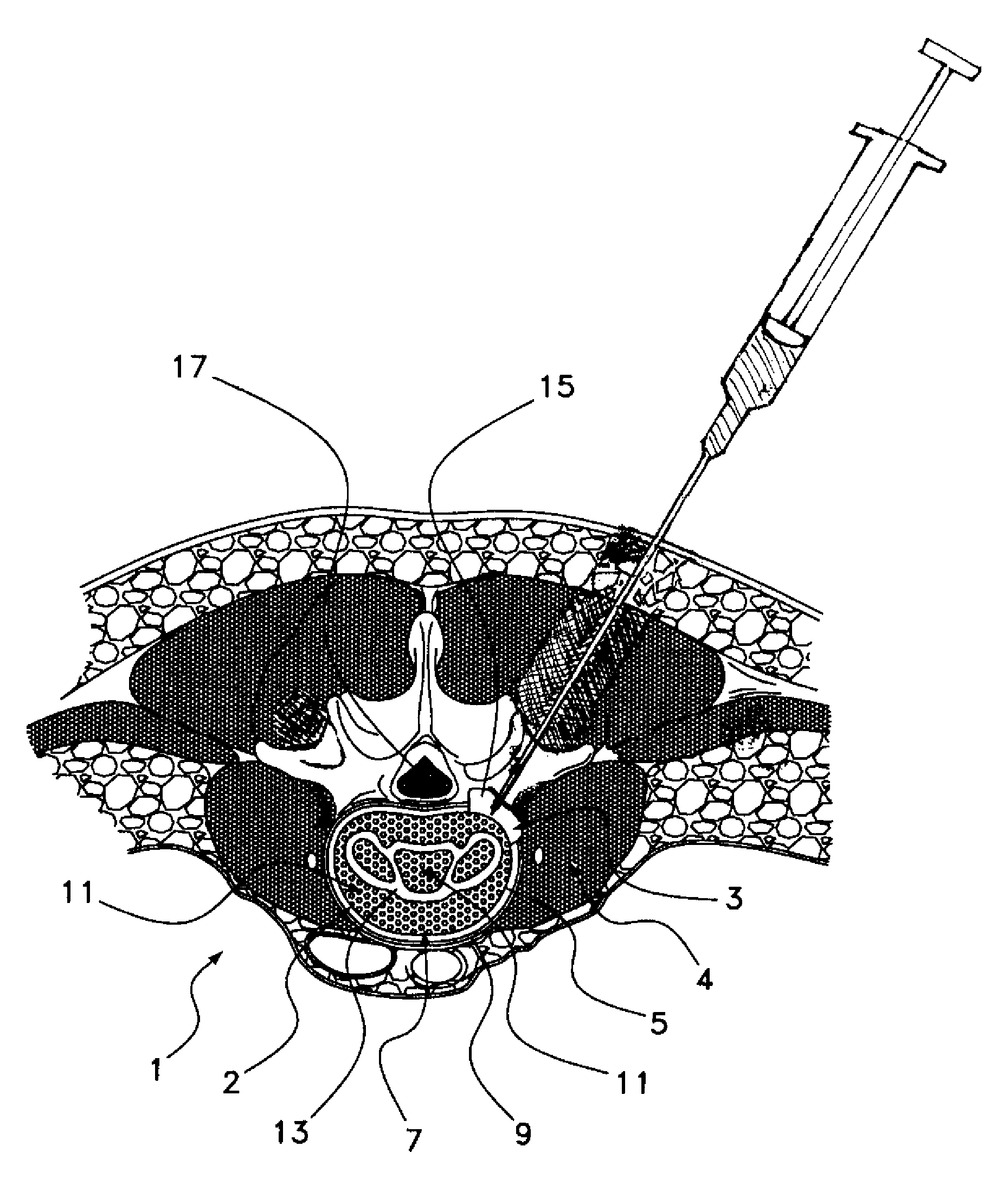
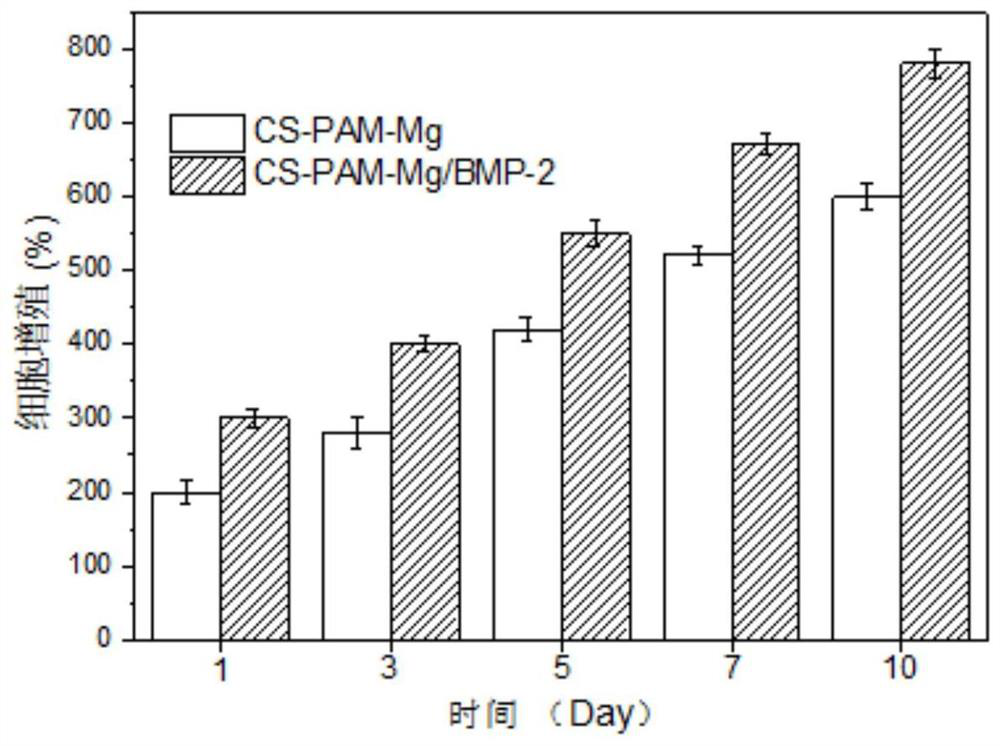
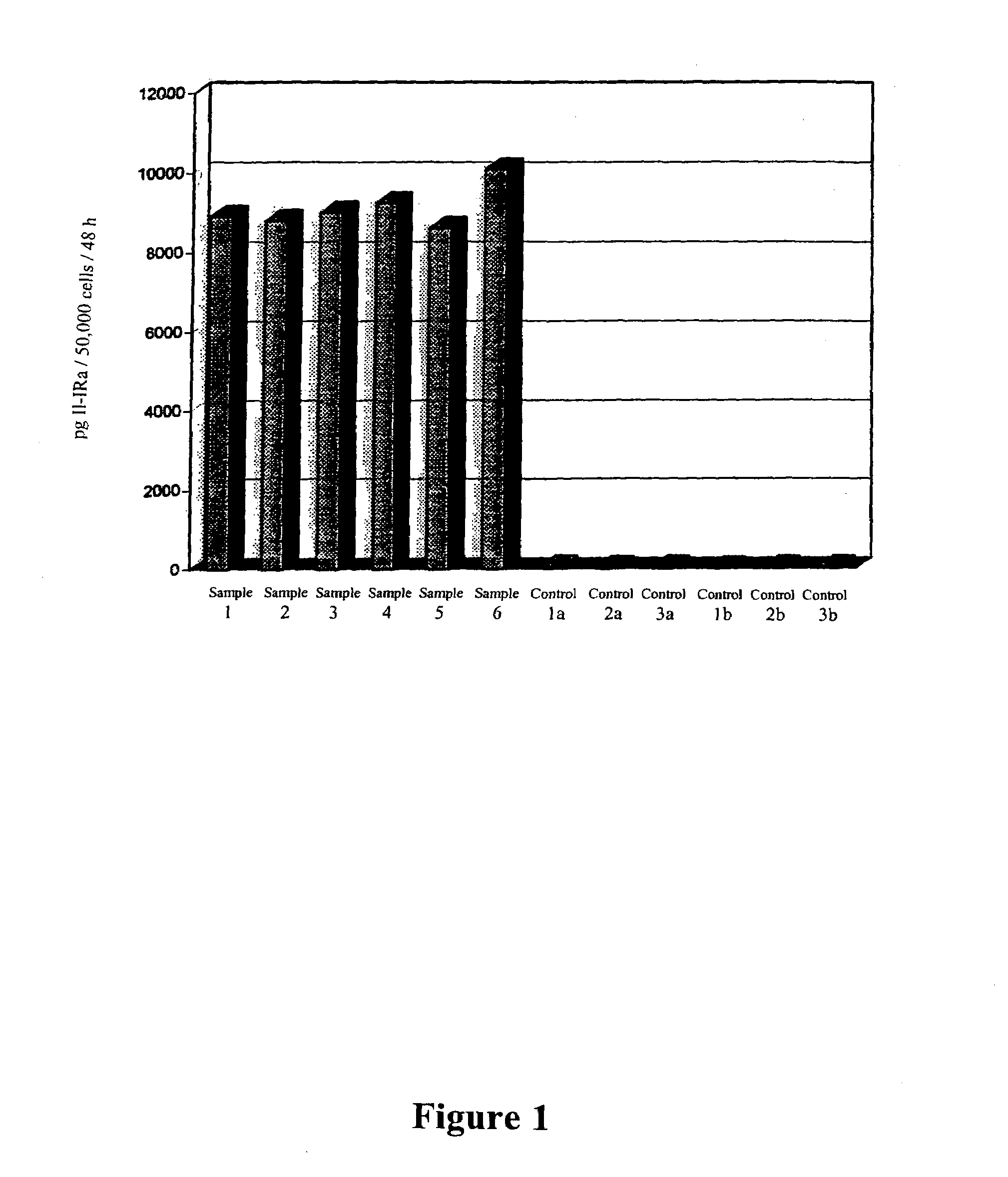
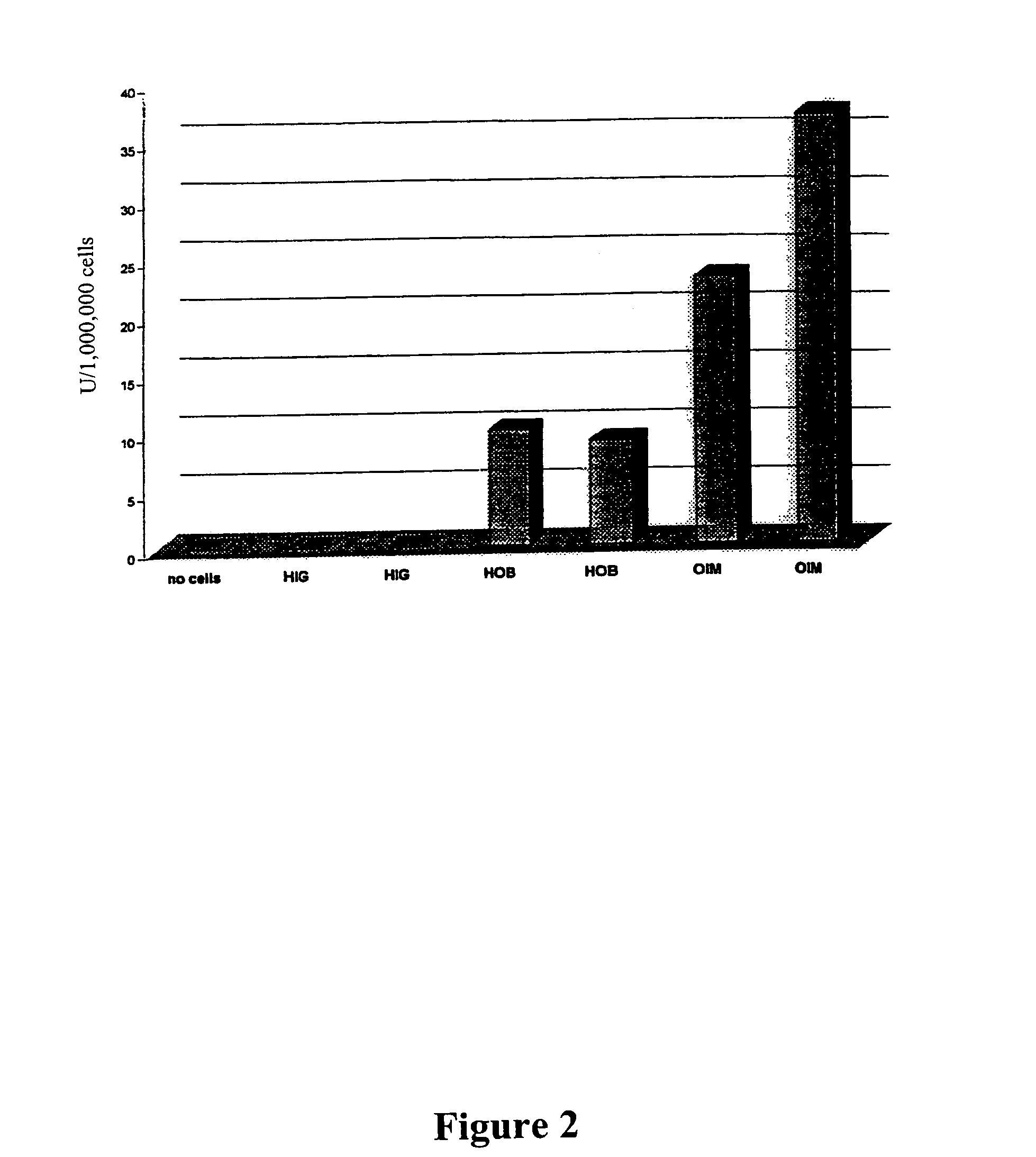
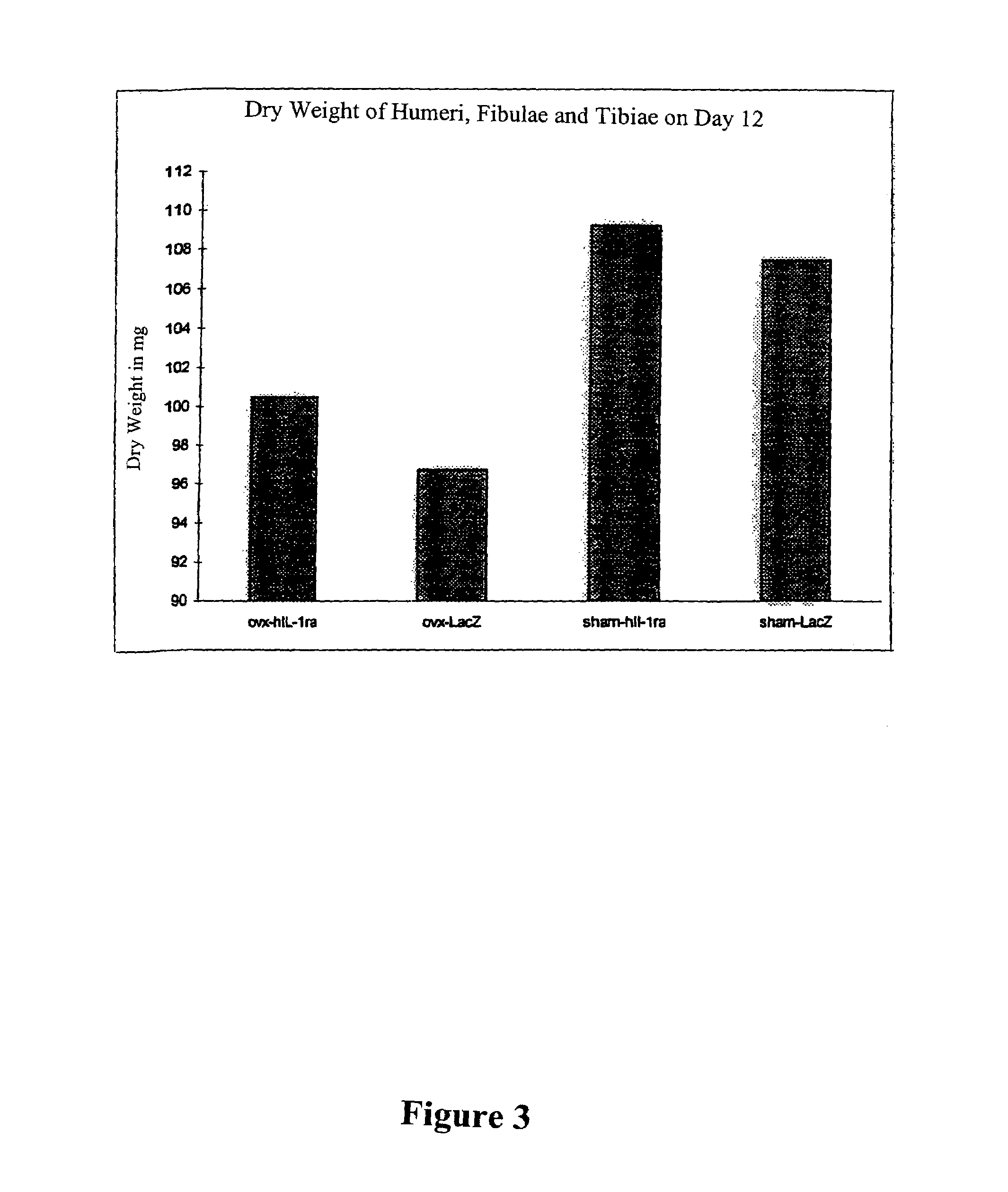
Patents
Literature
32 results about "OSTEOINDUCTIVE FACTOR" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Keywords: Diabetic nephropathy, osteoinductive factor, early diagnosis, biomarker Introduction Diabetic nephropathy (DN) is a diabetic syndrome called chronic capillaries pathological change and has become the leading cause of end stage renal diseases in diabetic patients from Asia, Europe and United States [ 1 , 2 ].
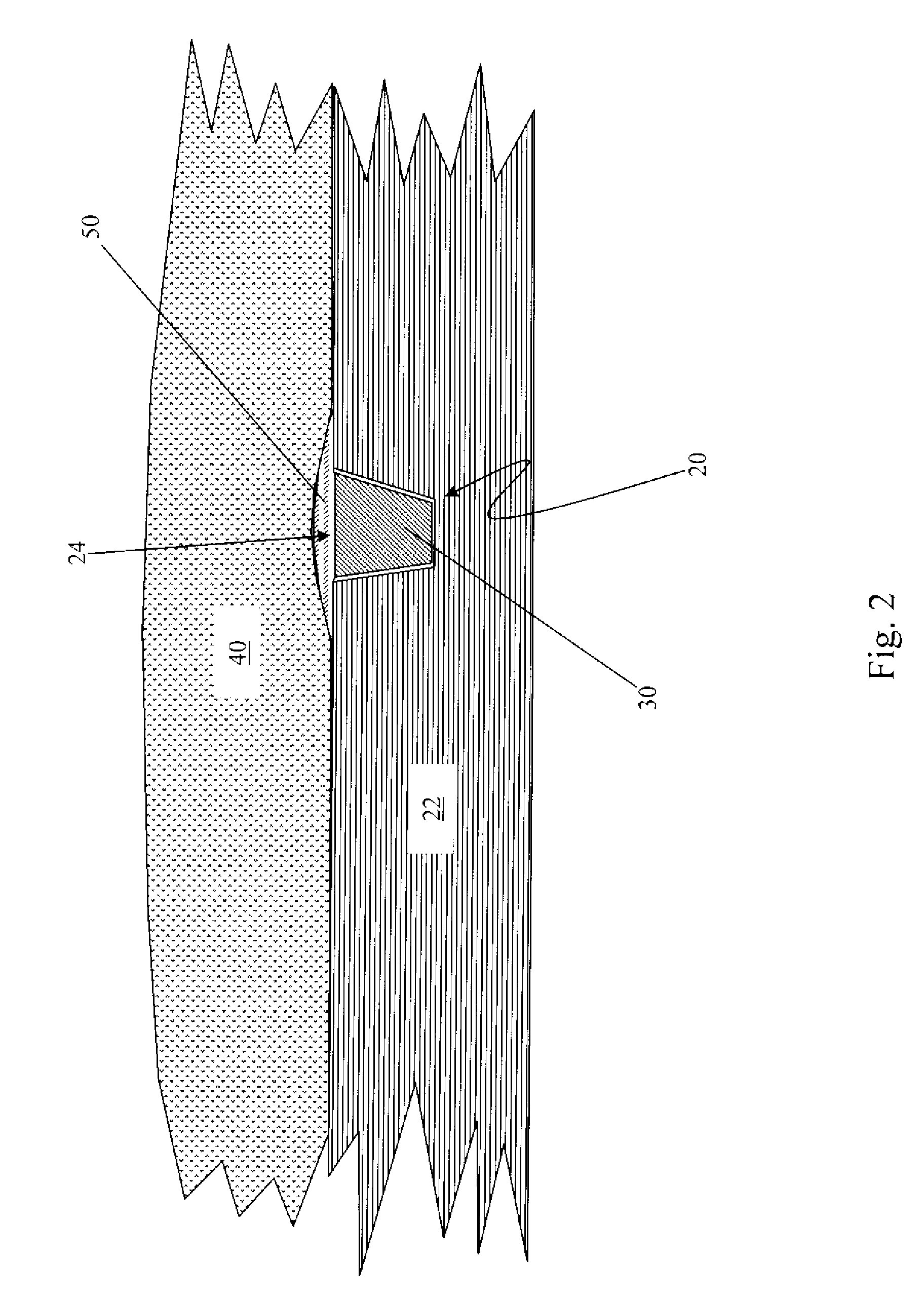
Bone Matrix Compositions and Methods
ActiveUS20070098756A1High activityEasy to addPeptide/protein ingredientsBone implantActive agentOSTEOINDUCTIVE FACTOR
An osteoinductive composition, corresponding osteoimplants, and methods for making the osteoinductive composition are disclosed. The osteoinductive composition comprises osteoinductive factors, such as may be extracted from demineralized bone, and a carrier. The osteoinductive composition is prepared by providing demineralized bone, extracting osteoinductive factors from the demineralized bone, and adding the extracted osteoinductive factors to a carrier. Further additives such as bioactive agents may be added to the osteoinductive composition. The carrier and osteoinductive factors may form an osteogenic osteoimplant. The osteoimplant, when implanted in a mammalian body, can induce at the locus of the implant the full developmental cascade of endochondral bone formation including vascularization, mineralization, and bone marrow differentiation. Also, in some embodiments, the osteoinductive composition can be used as a delivery device to administer bioactive agents.
Owner:WARSAW ORTHOPEDIC INC
Bone graft
ActiveUS7163691B2Fast reduction in osteoinductiveGood curative effectOrganic active ingredientsImpression capsOSTEOINDUCTIVE FACTORIn vivo
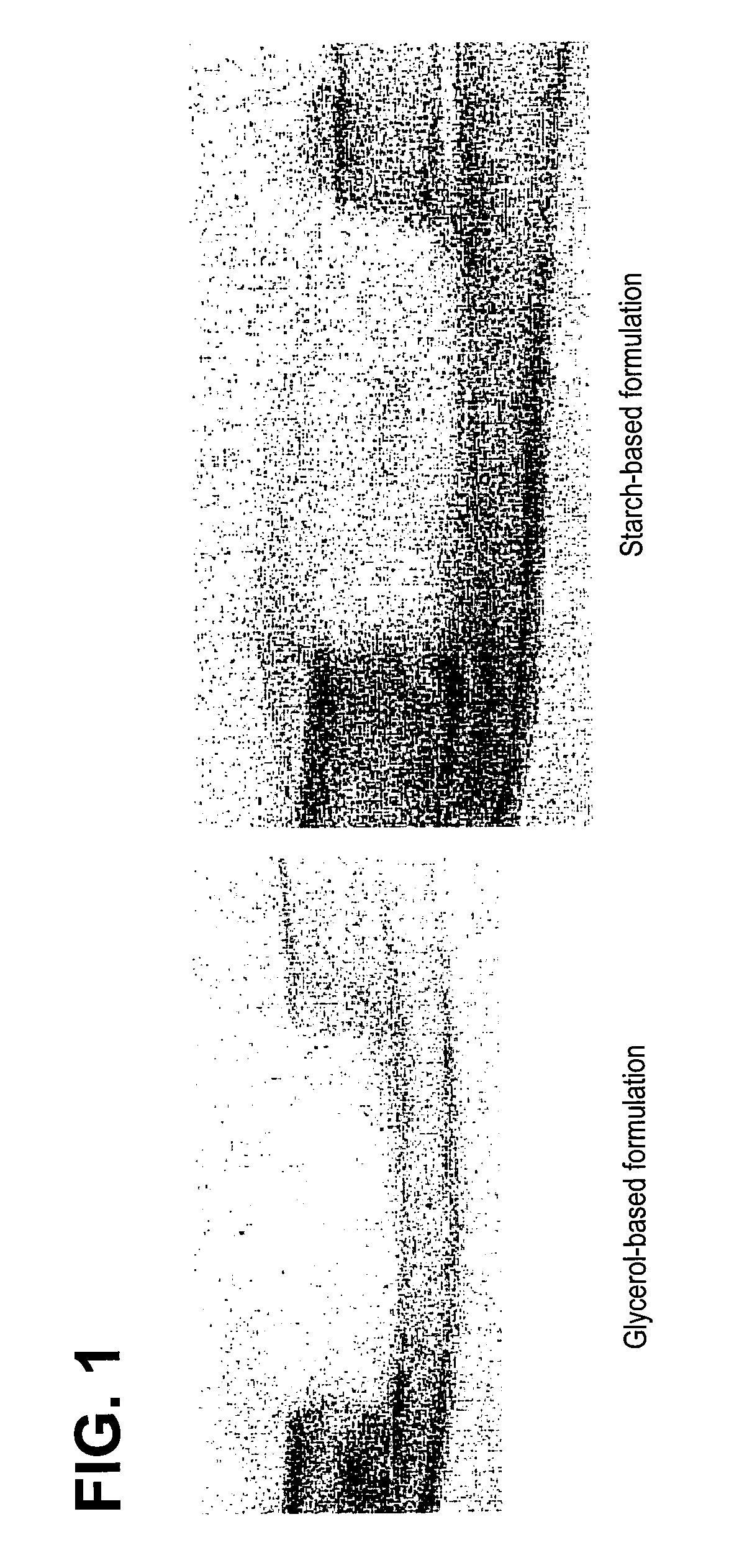
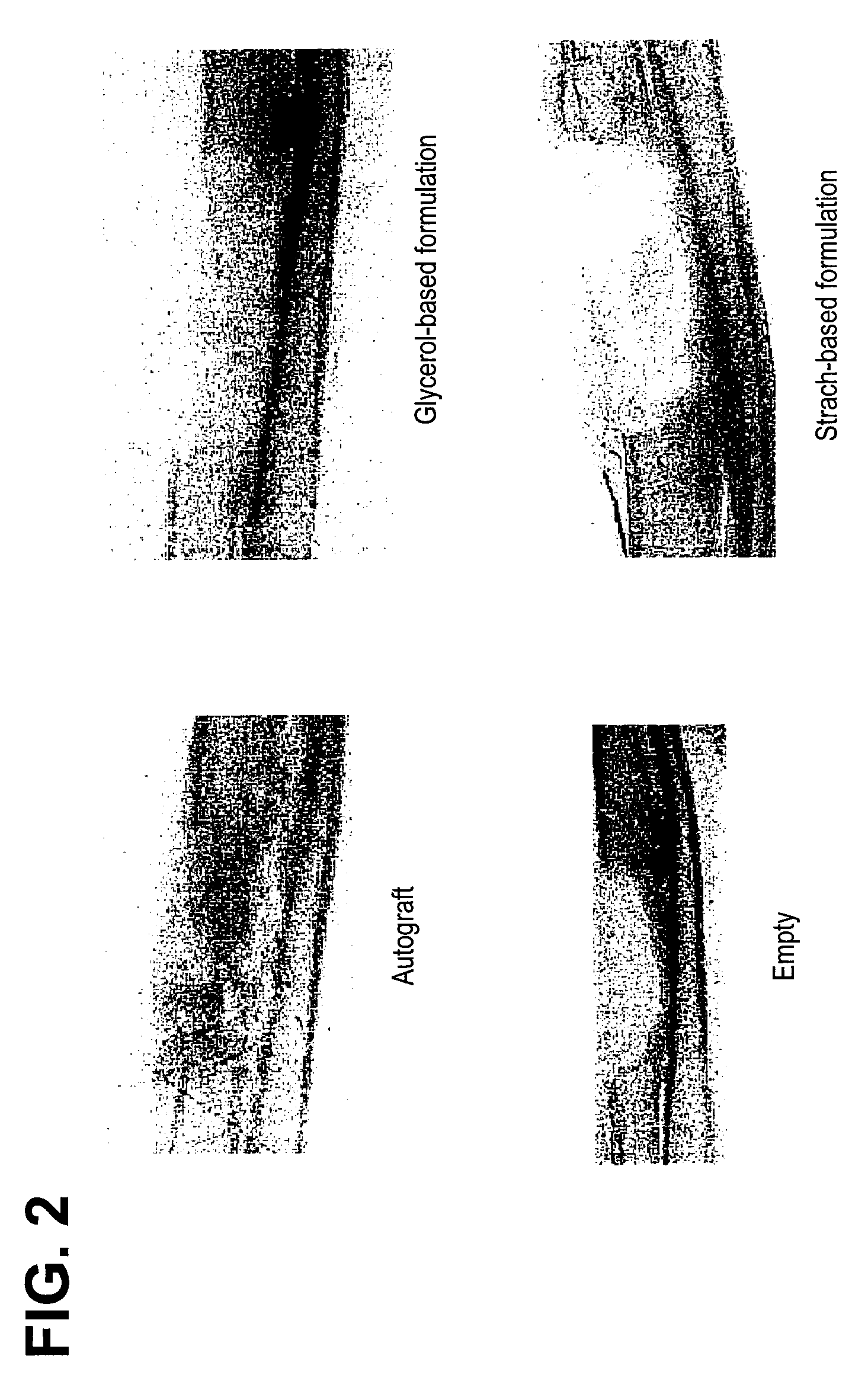
An improved demineralized bone matrix (DBM) or other matrix composition is provided that has been mixed with a stabilizing agent that acts as (1) a diffusion barrier, (2) a enzyme inhibitor, (3) a competitive substrate, or (4) a masking moiety. A diffusion barrier acts as a barrier so as to protect the osteoinductive factors found in DBM from being degraded by proteolytic and glycolytic enzymes at the implantation site. Stabilizing agents may be any biodegradable material such as starches, modified starches, cellulose, dextran, polymers, proteins, and collagen. As the stabilizing agents degrades or dissolves in vivo, the osteoinductive factors such as TGF-β, BMP, and IGF are activated or exposed, and the activated factors work to recruit cells from the preivascular space to the site of injury and to cause differentiation into bone-forming cells. The invention also provides methods of preparing, testing, and using the inventive improved osteodinductive matrix compositions.
Owner:WARSAW ORTHOPEDIC INC
Bone Matrix Compositions and Methods
ActiveUS20070110820A1High activityEasy to addPeptide/protein ingredientsImmunoglobulinsOSTEOINDUCTIVE FACTORBone implant
Owner:WARSAW ORTHOPEDIC INC
Bone Graft
ActiveUS20080145392A1Good curative effectSimple compositionAdditive manufacturing apparatusBone implantOSTEOINDUCTIVE FACTORIn vivo
An improved demineralized bone matrix (DBM) or other matrix composition is provided that has been mixed with a stabilizing agent that acts as (1) a diffusion barrier, (2) a enzyme inhibitor, (3) a competitive substrate, or (4) a masking moiety. A diffusion barrier acts as a barrier so as to protect the osteoinductive factors found in DBM from being degraded by proteolytic and glycolytic enzymes at the implantation site. Stabilizing agents may be any biodegradable material such as starches, modified starches, cellulose, dextran, polymers, proteins, and collagen. As the stabilizing agents degrades or dissolves in vivo, the osteoinductive factors such as TGF-.beta., BMP, and IGF are activated or exposed, and the activated factors work to recruit cells from the preivascular space to the site of injury and to cause differentiation into bone-forming cells. The invention also provides methods of preparing, testing, and using the inventive improved osteodinductive matrix compositions
Owner:WARSAW ORTHOPEDIC INC
Method of using an Anti-growth matrix as a barrier for cell attachment and osteo-inductive factors
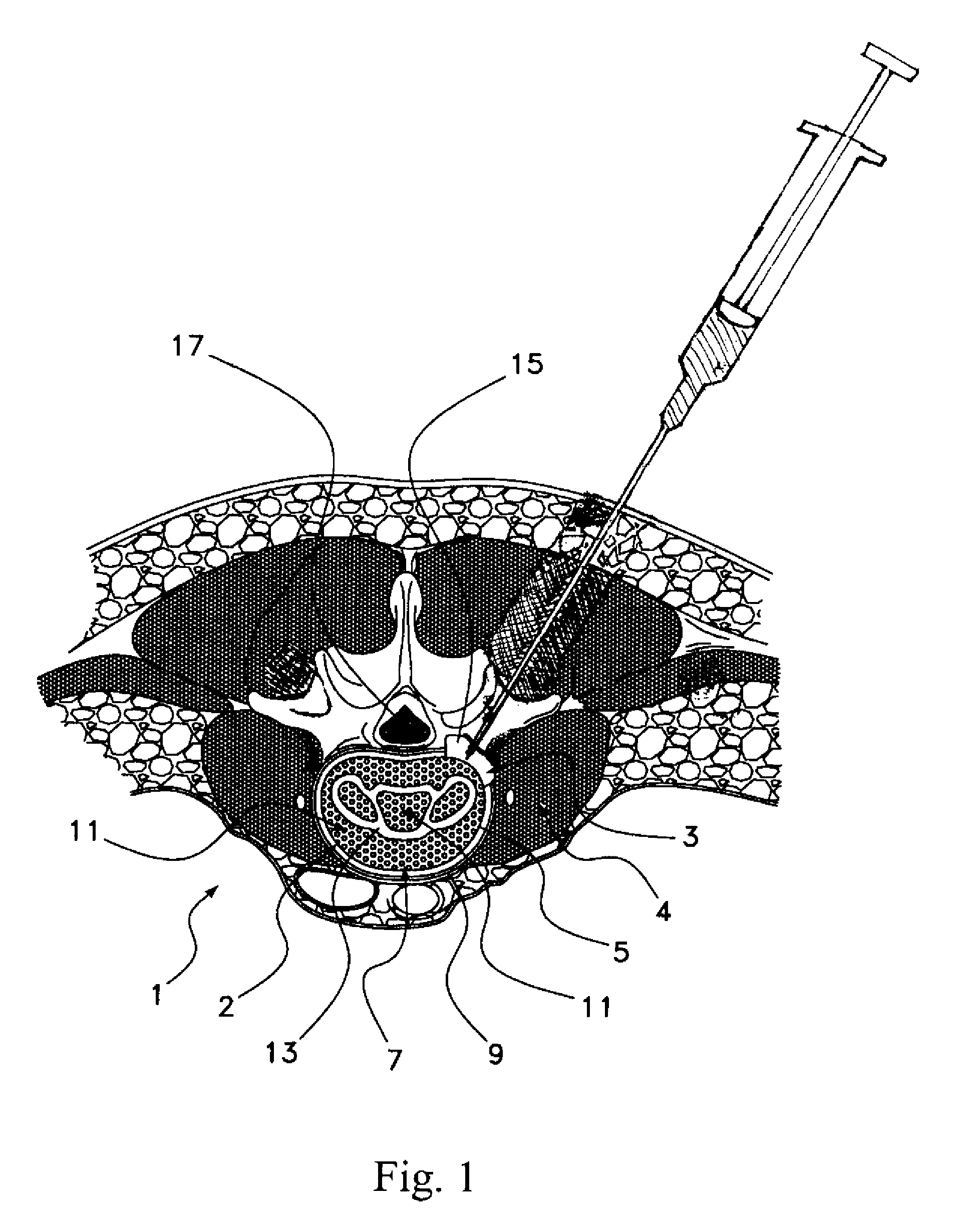
The present invention generally relates to a method of using a matrix as a barrier for unwanted cell attachment and bone formation in unwanted areas of the human body during implant procedures. More specifically, a growth-inhibiting matrix may be used to prevent migration of osteo-inductive agents or bone tissue from an intervertebral disc space through the outer bands of annulus fibrosis that abuts the spinal tissue, canal, and other surrounding areas.
Owner:WARSAW ORTHOPEDIC INC
Degradable bone lacuna inside haemostatic material and preparation method thereof
InactiveCN101444635ANon-irritatingGood biocompatibilityAbsorbent padsBandagesParticulatesOSTEOINDUCTIVE FACTOR
The present invention discloses a kind of degradable bone lacuna inside haemostatic material and preparation method thereof which belongs to biological material field. The purpose of the invention is to solve existing problem that bone wax not easily degraded and absorbed, or degradation production tend to initiate inflammation. The haemostatic material described in the invention is composed of 1-10% medical sodium alginate solution in mass concentration and medical faecula which accounts for 15-40% of mass of sodium alginate solution. Inorganic particulate, antibiotic, bone-inducing factor and haemostatic can be further added. Degradable bone lacuna inside haemostatic material can be prepared by mixing medical sodium alginate solution and medical faecula. The haemostatic material of the invention is easy degraded, the degradation production is innoxious and nonirritant to human organisms, and has good ductibility and mechanical strength.
Owner:BEIJING UNIV OF CHEM TECH +1
Biological activity bone renovation material with bone-inducing factor control-release function and production method thereof
InactiveCN101219241AAvoid the influence of biological activityEvenly distributedProsthesisMicrosphereBiocompatibility Testing
The invention discloses a nano bone repair material with biological activity and a preparation method thereof. The nano bone repair material with biological activity has controlled release effectiveness for bone inductive factors and belongs to the technical field of biomedical materials. The bone repair material with biological activity consists of nano-hydroxyapatite / collagen composite nHAC, biodegradable polymer material polylactic acid PLA and chitosan controlled release microspheres for treating effective quantity of bone inductive factors. By adopting the method described in the patent claim 1 of physically mixing three materials and combining the three materials by supersonic dispersion technology, the bone repair material with biological activity is compounded and prepared. The invention is a novel bone which has excellent biocompatibility and biodegradability and better bone inductive property than bone inductive property reported in technical literature reports of the prior art, and is very hopeful to be widely used in clinic as the repair material of bone defects.
Owner:TSINGHUA UNIV
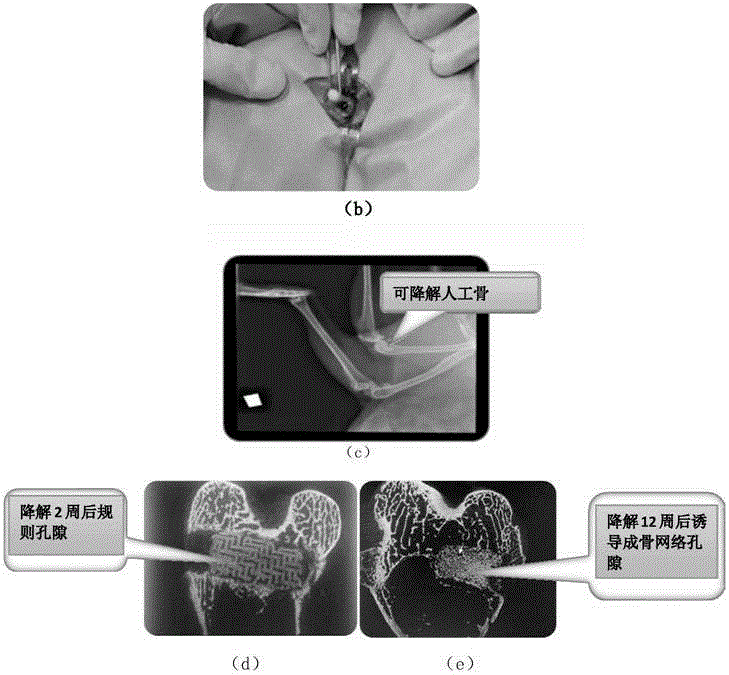
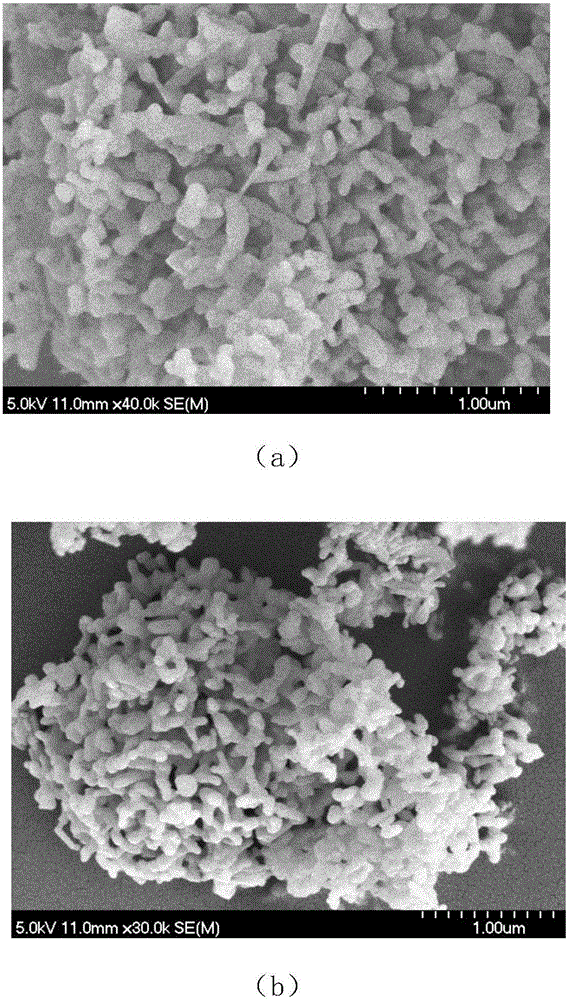
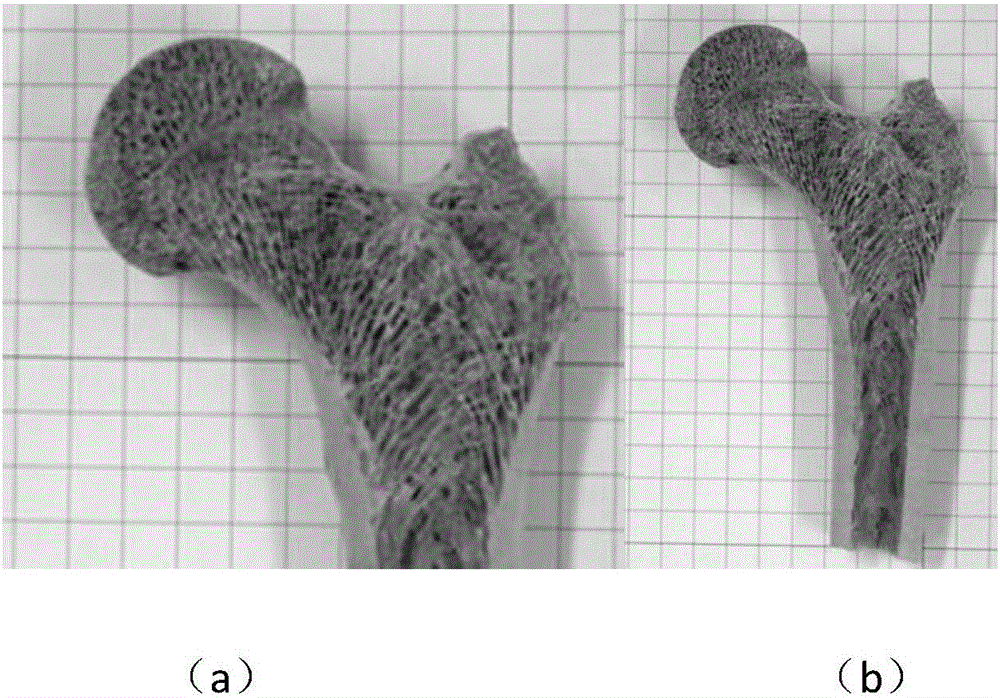
Ceramic matrix degradable artificial bone biomaterial for 3D printing
InactiveCN105770996AFavorable for attachment growthHigh porosityAdditive manufacturing apparatusTissue regenerationOSTEOINDUCTIVE FACTORTri calcium phosphate
The invention discloses a ceramic matrix degradable artificial bone biomaterial for 3D printing. The biomaterial is formed by compounding a bone morphogenetic protein, tricalcium phosphate, biological ceramic and polylactic acid in percentage by weight, wherein for different age groups, the weight percentages are different. According to the preparation method, the non-wire printing is adopted, the biological ceramic and tricalcium phosphate powder are mixed uniformly, furthermore, the polylactic acid and the bone morphogenetic protein are canned in a liquid state, mixed powder enters a printer by being absorbed through a vacuum guiding pipe of the printer, the polylactic acid (PLA) and the bone morphogenetic protein (BMP) which are canned in the liquid state are fully mixed with the mixed powder to form a polymer, and then a complex artificial bone biomaterial model is printed through a printer head.
Owner:金马丁明
Spinal fusion methods and devices
InactiveUS20090298777A1Reduce formationPromote and limit bone growthPeptide/protein ingredientsBone implantPosterior approachOSTEOINDUCTIVE FACTOR
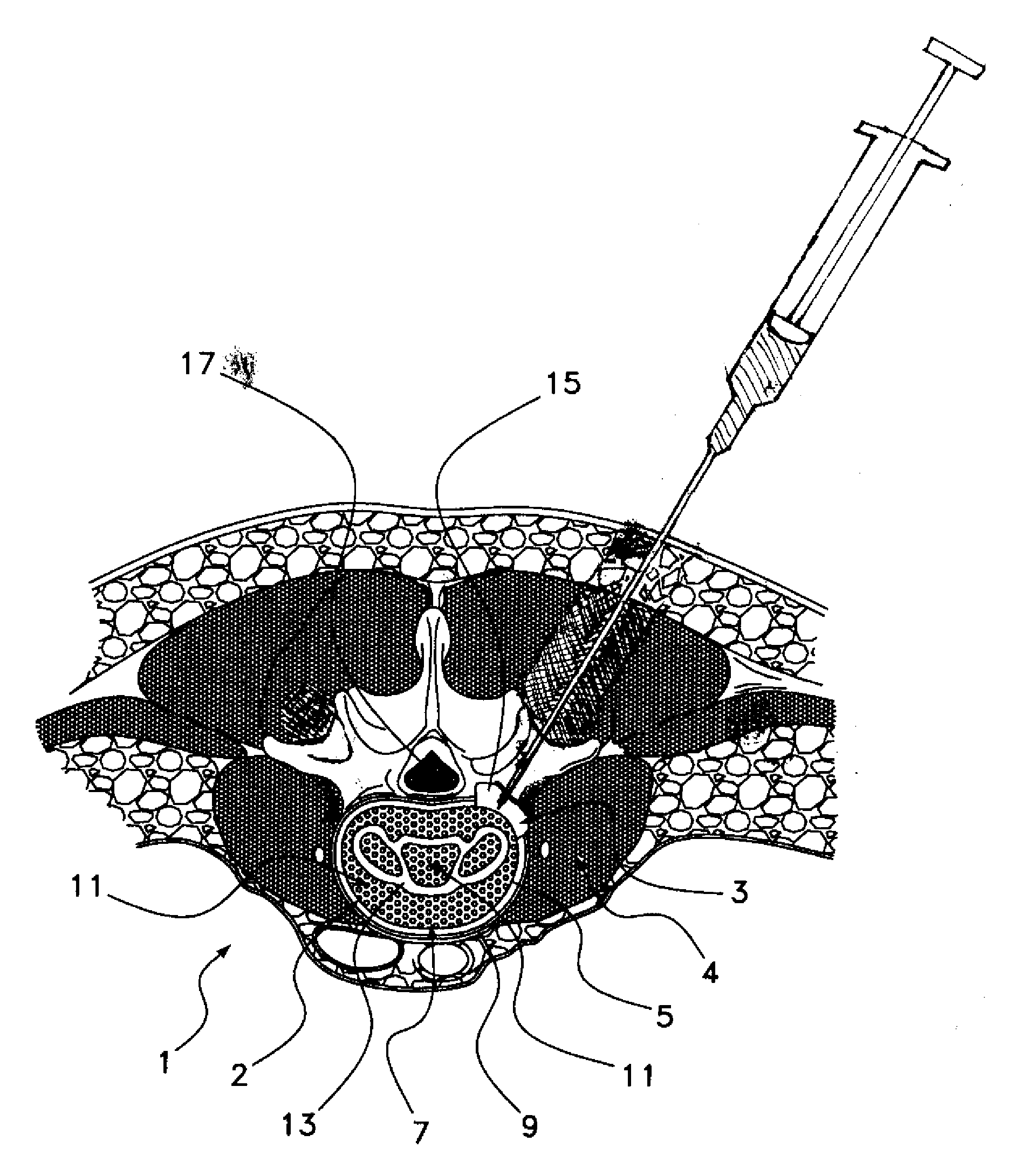
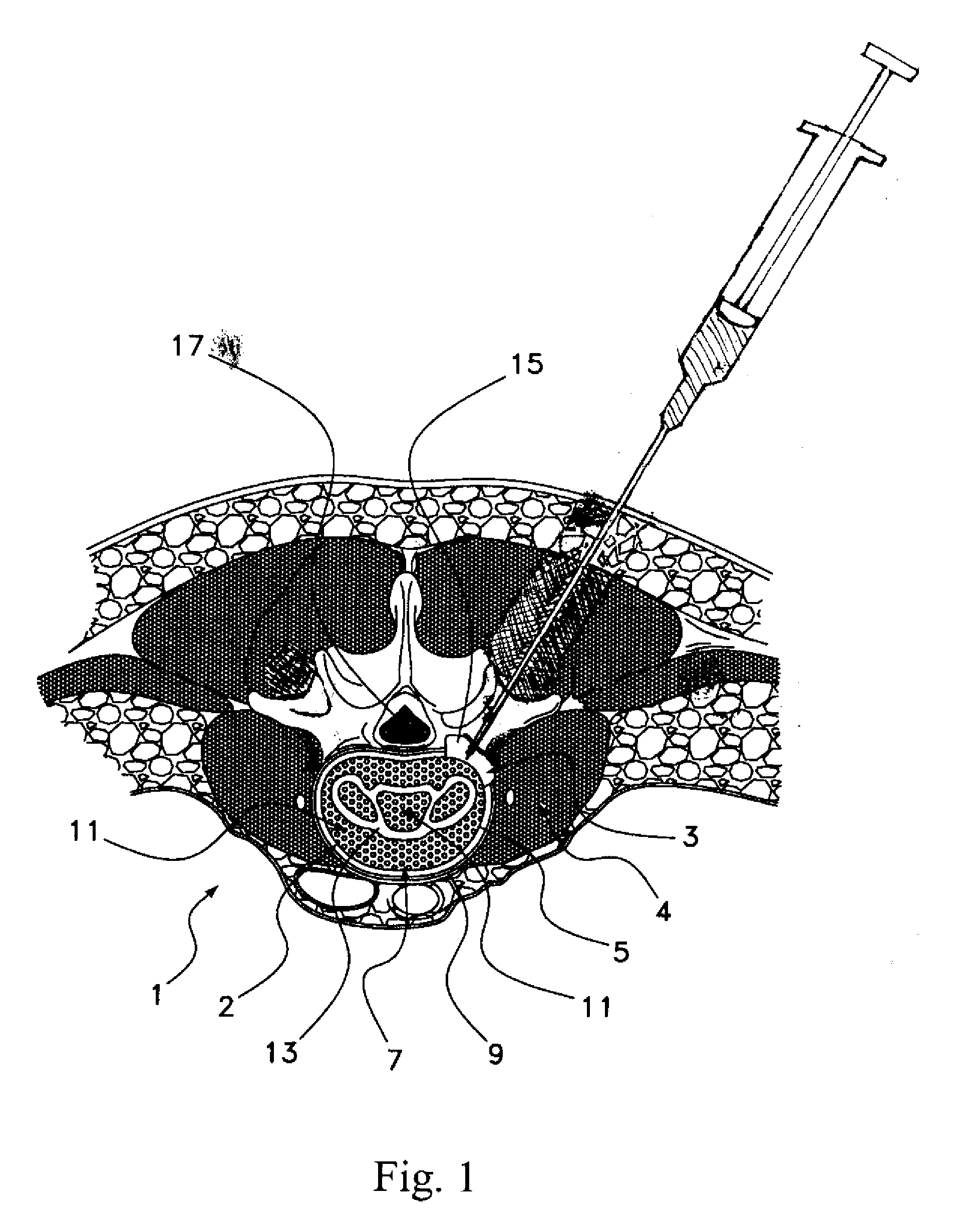
Methods, devices and compositions for fusing adjacent vertebrae, and otherwise localizing bone growth, are provided. In one form of the invention, a method for fusing adjacent vertebrae includes preparing a disc space for receipt of an intervertebral disc implant in an intervertebral disc space between adjacent vertebrae, inserting the implant into the intervertebral disc space and providing an osteoinductive composition that includes an osteoinductive factor in a pharmaceutically acceptable carrier. The carrier is advantageously substantially impermeable to efflux of the osteoinductive factor and is released as the carrier is resorbed or biodegraded. Preferred carriers include a hardened, resorbable carrier, such as a calcium phosphate cement that retains at least about 50% of the osteoinductive factors greater than about 2 days. Preferred osteoinductive factors are growth factors and include bone morphogenctic proteins and LIM mineralization proteins. In alternative forms of the invention, the method may be performed without utilization of a load-bearing spinal implant by disposing the osteoinductive composition in the disc space. The method is advantageously performed on lumbar vertebrae by a posterior approach. Intervertebral fusion devices and methods for their preparation are also provided.
Owner:WARSAW ORTHOPEDIC INC
Method of using an anti-growth matrix as a barrier for cell attachment and osteo-inductive factors
Owner:WARSAW ORTHOPEDIC INC
Injectable hydrogel for promoting bone regeneration and preparation method of injectable hydrogel
InactiveCN111658820AGood injectabilityEasy injectionPharmaceutical delivery mechanismTissue regenerationMethacrylateMagnesium phosphate
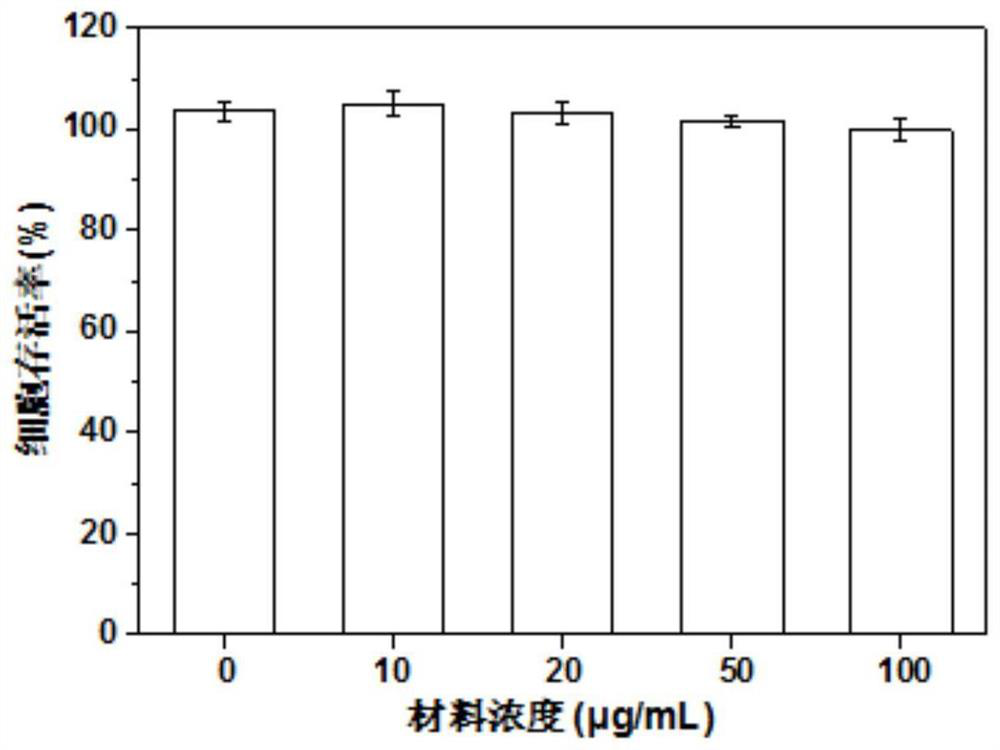
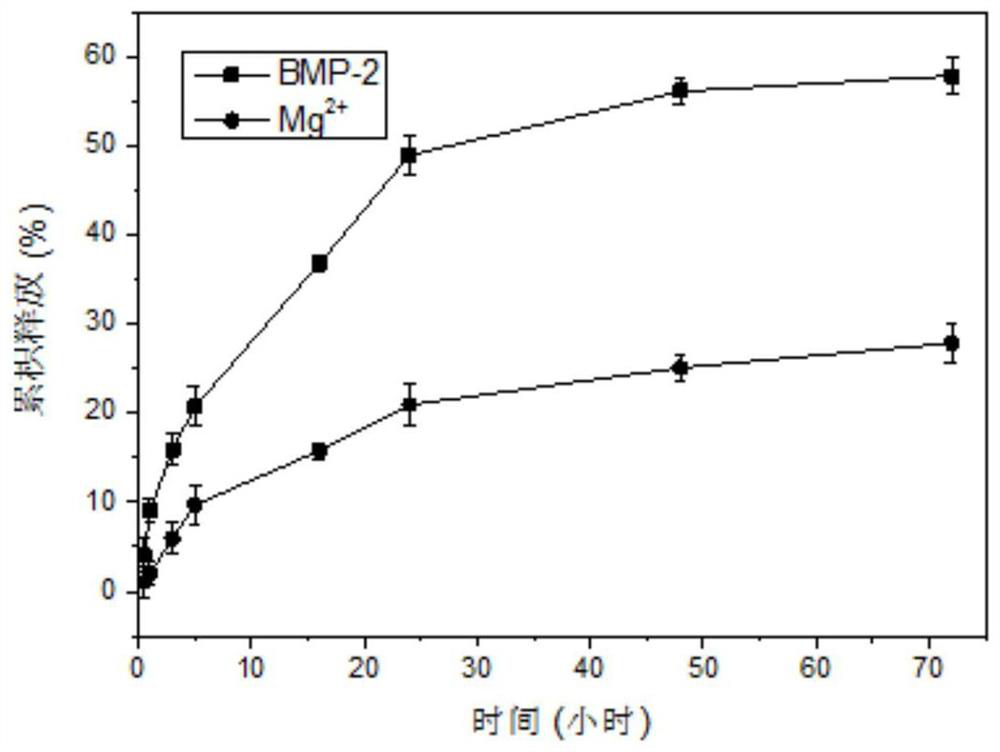
The invention relates to injectable hydrogel for promoting bone regeneration and a preparation method of the injectable hydrogel, and belongs to the technical field of biomedical engineering materials. The invention provides a bioactive nano-composite hydrogel based on chitosan methacrylate and pami magnesium phosphate nano-particles. The hydrogel is used for local delivery and on-demand simultaneous release of bioactive ions and biological factors. The nano-composite hydrogel prepared by the invention has good injectability and effective stress relaxation, so that the hydrogel is easy to inject and adapts to bone defects with irregular shapes. Magnesium ions released by the hydrogel promote osteogenic differentiation of human mesenchymal stem cells (hMSCs) and activation of alkaline phosphatase (ALP). In addition, a bone induction factor BMP-2 is released to further promote osteogenic differentiation of the hMSC. Results show that the injectable nano-composite hydrogel mediates optimized release of various treatment factors, and effectively promotes in-situ bone regeneration through minimally invasive surgery.
Owner:GUANGZHOU CHUANGSAI BIOLOGICAL MEDICAL MATERIALS CO LTD
Viral and non-viral vectors as vehicles for delivering transgenes for treating bone pathologies
The present invention relates to a method for treating bone pathologies comprising delivering a viral or non-viral delivery vehicle comprising genetic information (e.g. a transgene) encoding a therapeutic osteoinductive factor to target cells in vivo enabling the cells to produce the osteoinductive factor at the site of the bone pathology. The delivery is achieved by a simplified method which does not require cumbersome ex vivo techniques or additional matrix or scaffolding agents. Such viral and non-viral delivery vehicles of the present invention are derived from the following nonlimiting examples: adenoviruses, adeno-associated viruses, retroviruses, herpes simplex viruses, liposomes, and plasmids. The osteoinductive factors include, but are not limited to, growth factors, cytokines, growth factor inhibitors and cytokine inhibitors.
Owner:UNIVERSITY OF PITTSBURGH
Viral and non-viral vectors as vehicles for delivering transgenes for treating bone pathologies
The present invention relates to a method for treating bone pathologies comprising delivering a viral or non-viral delivery vehicle comprising genetic information (e.g. a transgene) encoding a therapeutic osteoinductive factor to target cells in vivo enabling the cells to produce the osteoinductive factor at the site of the bone pathology. The delivery is achieved by a simplified method which does not require cumbersome ex vivo techniques or additional matrix or scaffolding agents. Such viral and non-viral delivery vehicles of the present invention are derived from the following nonlimiting examples: adenoviruses, adeno-associated viruses, retroviruses, herpes simplex viruses, liposomes, and plasmids. The osteoinductive factors include, but are not limited to, growth factors, cytokines, growth factor inhibitors and cytokine inhibitors.
Owner:BALTZER AXEL W +2
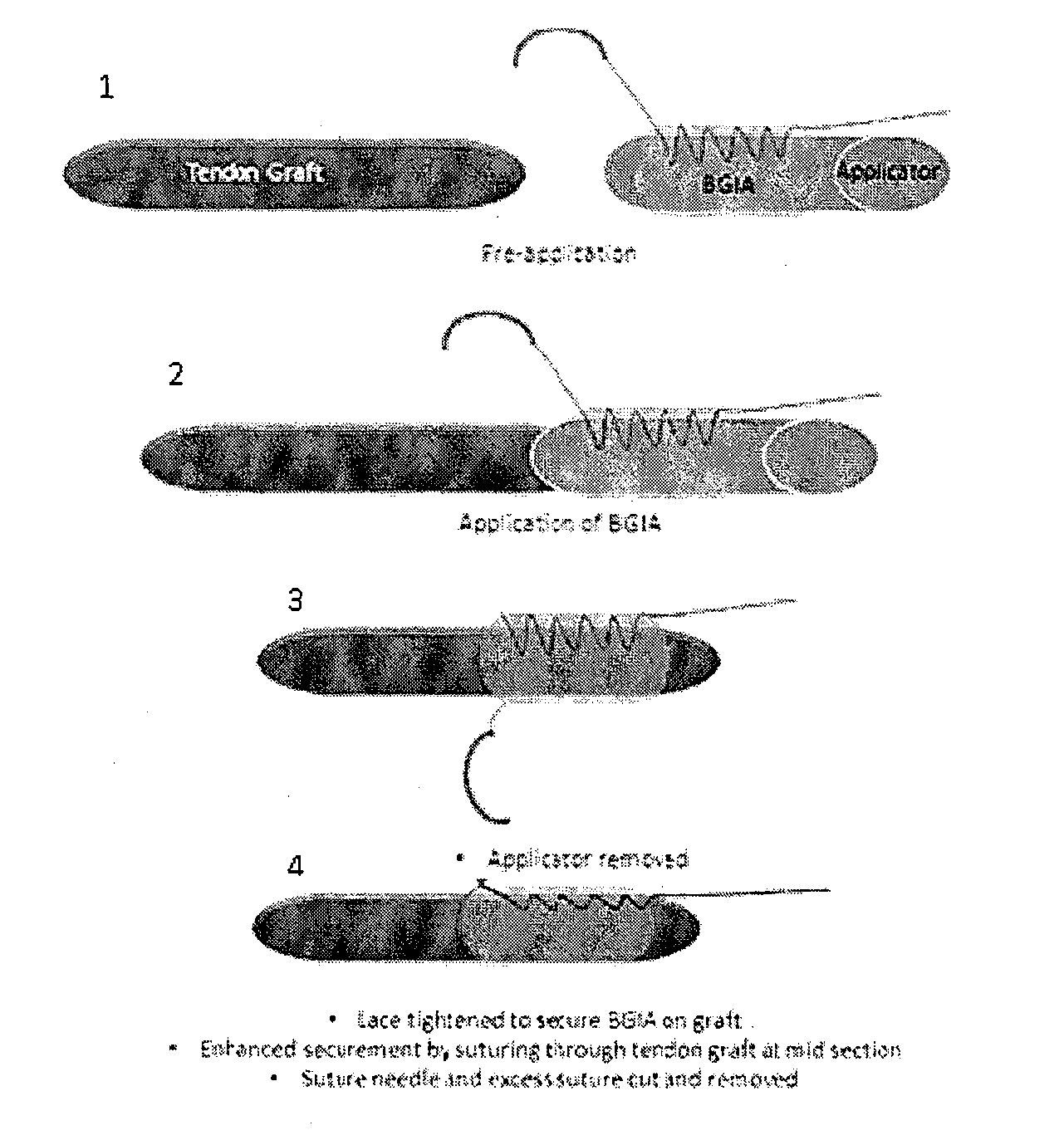
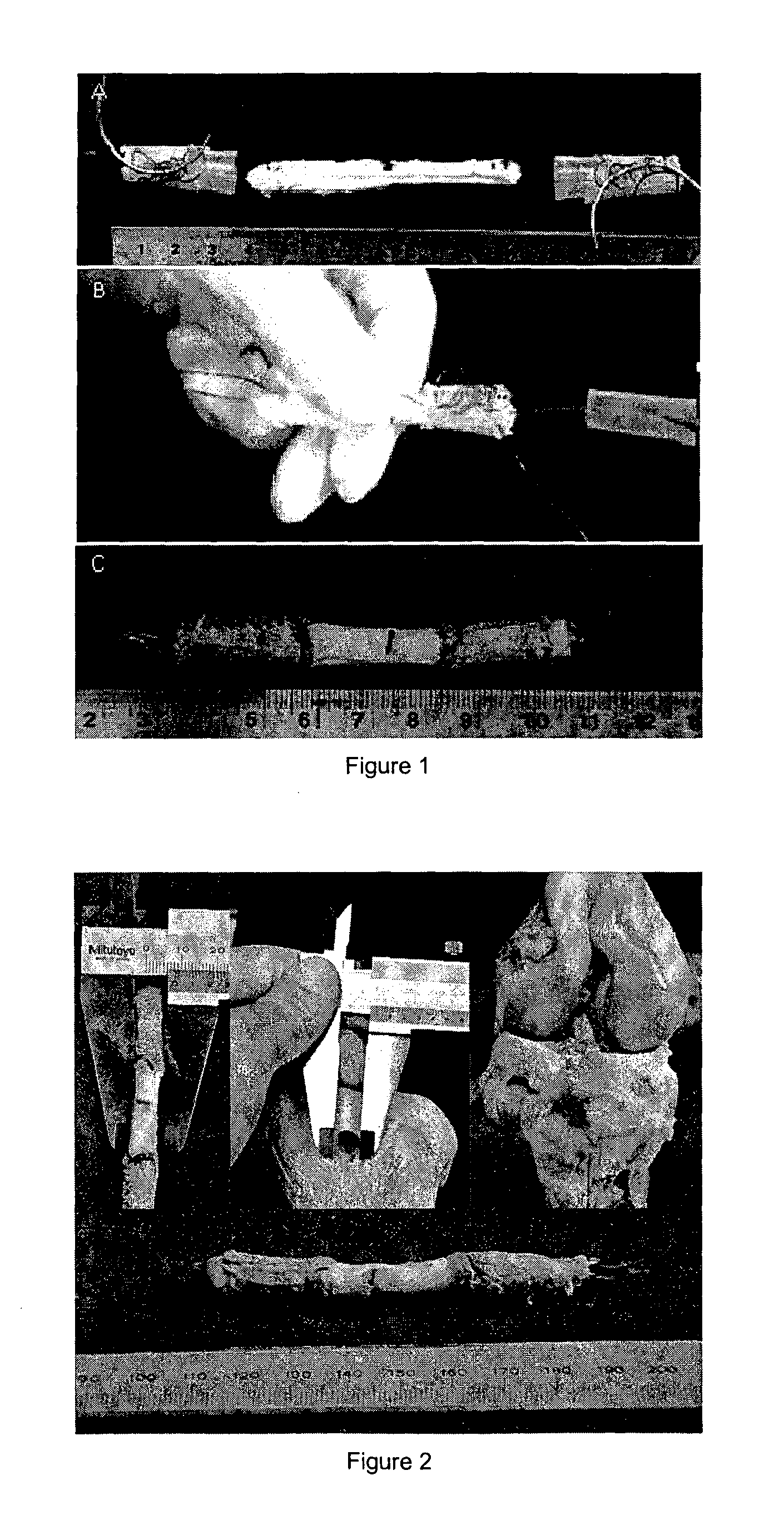
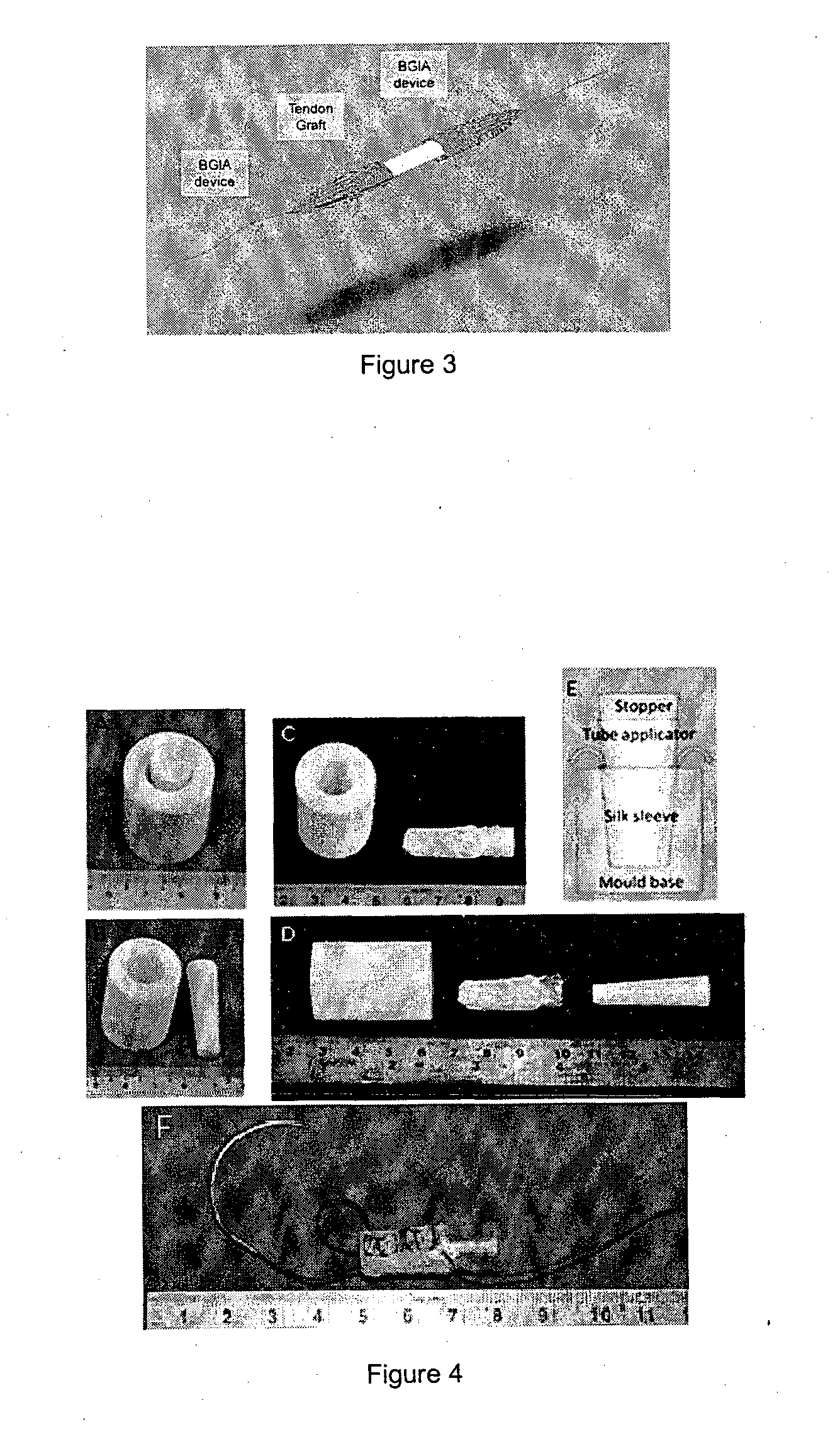
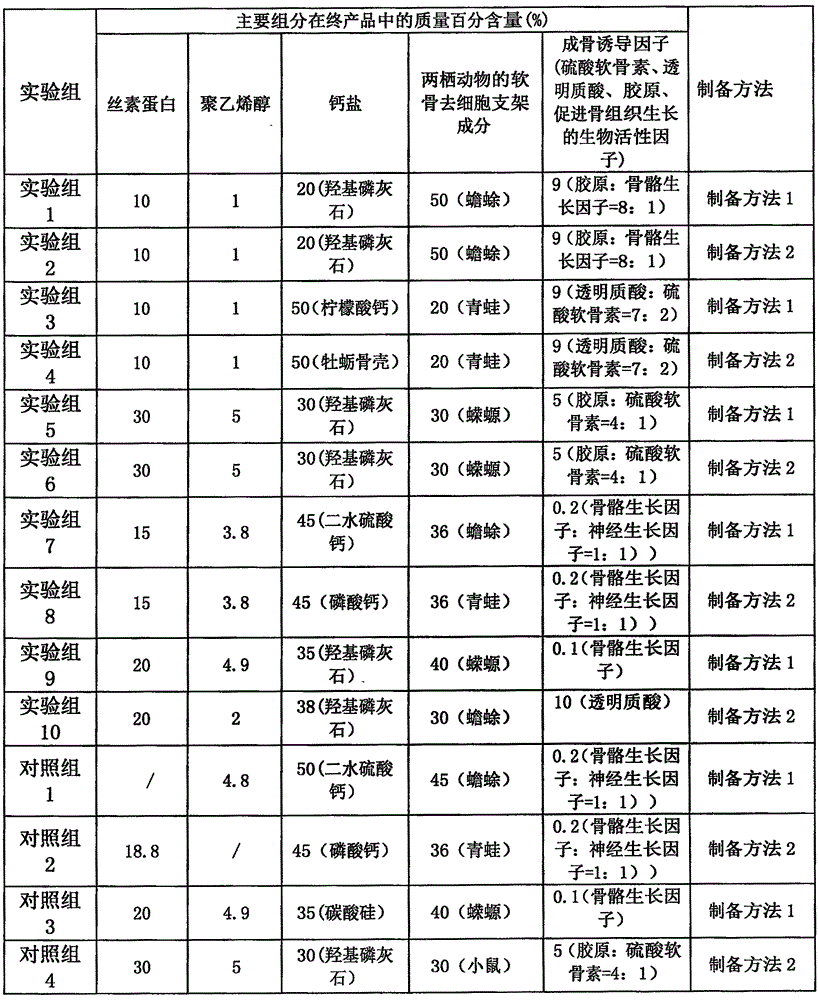
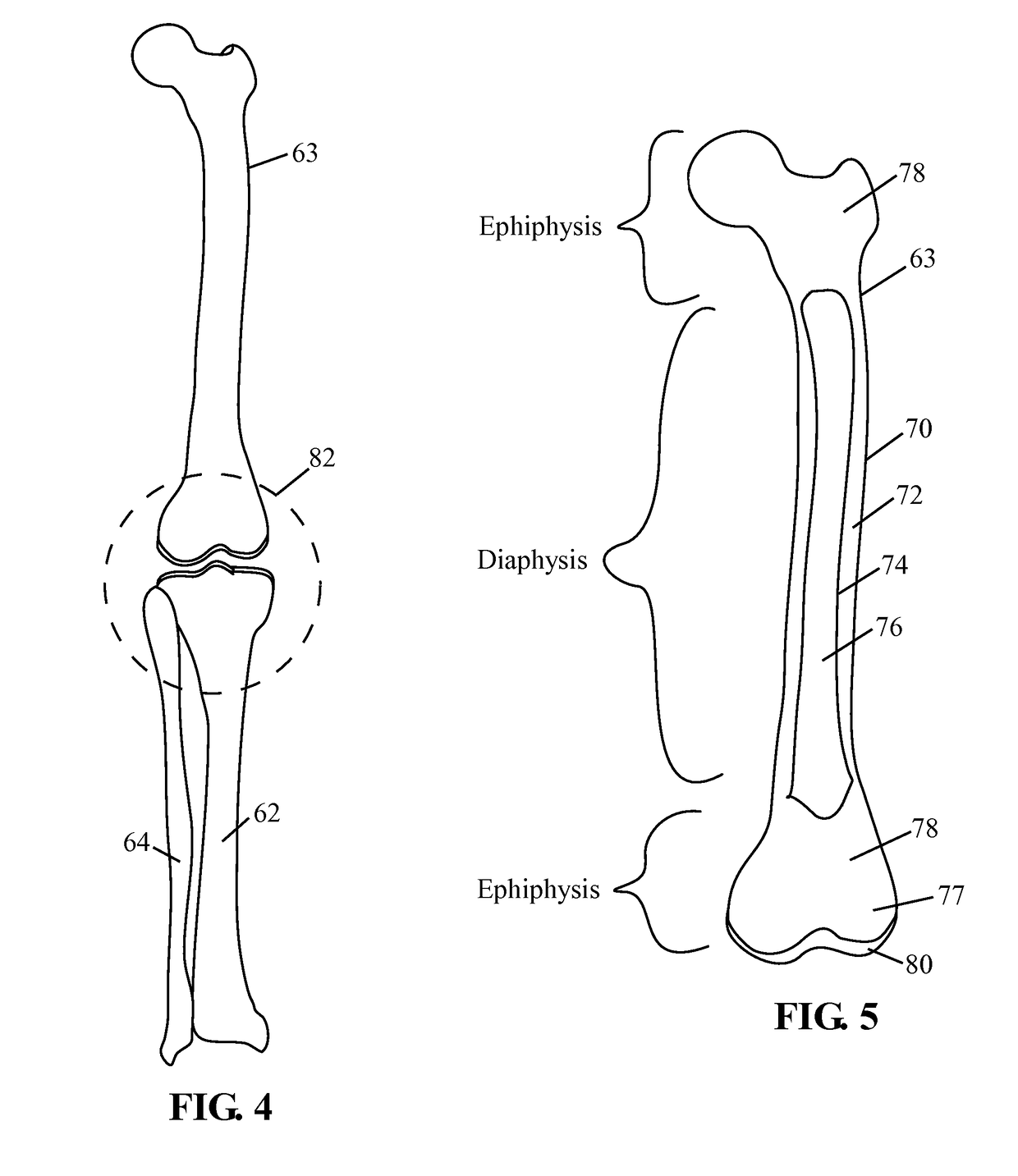
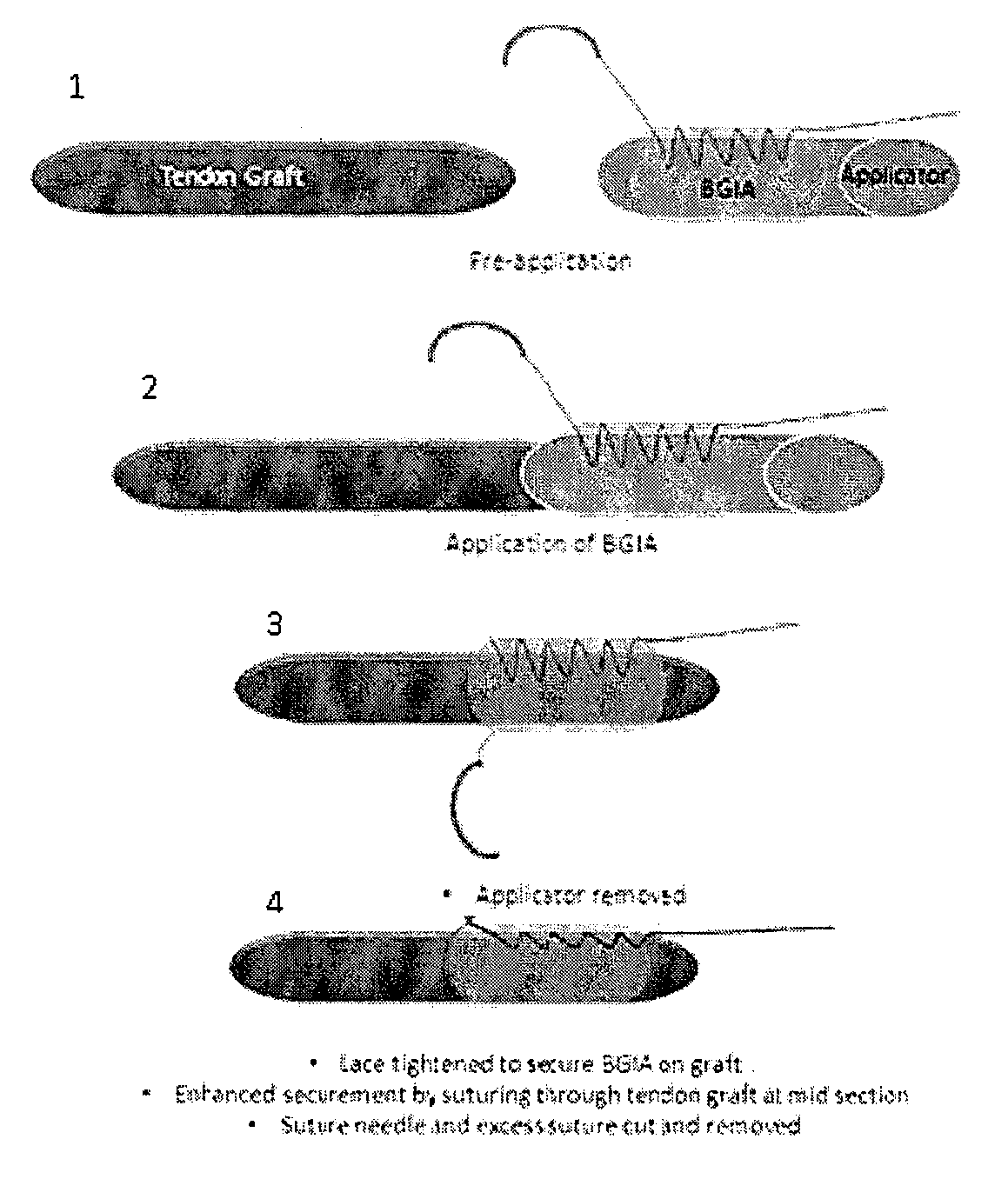
Tissue interface augmentation device for ligament/tendon reconstruction
ActiveUS20160157992A1Promote reconstructionPromote osseointegrationLigamentsMusclesFiberOSTEOINDUCTIVE FACTOR
The present invention relates to a device for the interfacial augmentation of tissue grafts for the purpose of tendon and ligament reconstruction. The inventive device works both as a delivery vessel for osteoconductive and osteoinductive factors and as a scaffold to stimulate and support bone ingrowth and comprises a tubular composite silk sheath, said tubular composite silk sheath comprising: a backbone consisting of a tubular silk mesh, and a carrier material consisting of a porous silk sponge, wherein said tubular silk mesh consists of degummed silk fibroin fibers, wherein said porous silk sponge comprises silk fibroin fibers and hydroxyapatite particles and said tubular silk mesh and said porous silk sponge form a composite material. The present invention is also directed to a method for the manufacturing of such augmentation devices, a method for fixation of the thus fabricated tissue interface augmentation devices onto ligament or tendon grafts, a method for applying such devices for ligament and / or tendon reconstruction to tendon grafts, as well as their application in ligament and tendon reconstruction.
Owner:NAT UNIV OF SINGAPORE +1
Biocompatible bone graft and preparation method thereof
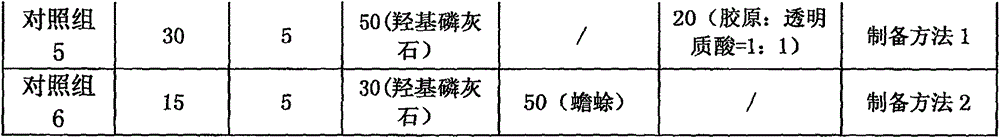
ActiveCN106492281AWide variety of sourcesIncrease elastic strengthTissue regenerationProsthesisCartilage cellsPolyvinyl alcohol
The invention provides a biocompatible bone graft. The main components of the stent include silk fibroin, polyvinyl alcohol, calcium salt, cartilage cytoskeleton-removed component of amphibian and an osteogenic induction factor; and the mechanical strength and degradation speed of the bone graft can be controlled by changing the proportion of the components. The biocompatible bone graft is of a three-dimensional solid shape and has good mechanical properties; and dense holes in the surface promote the adhesion and regeneration of cartilage cells. In the preparation process of the biocompatible bone graft, heating is not needed, no chemical or enzyme crosslinking agent is used, and the activity of each component is maintained. The biocompatible bone graft gives play to the collaborative advantage of each component and is applied to the substitute graft of bone tissue defect.
Owner:WENZHOU MEDICAL UNIV
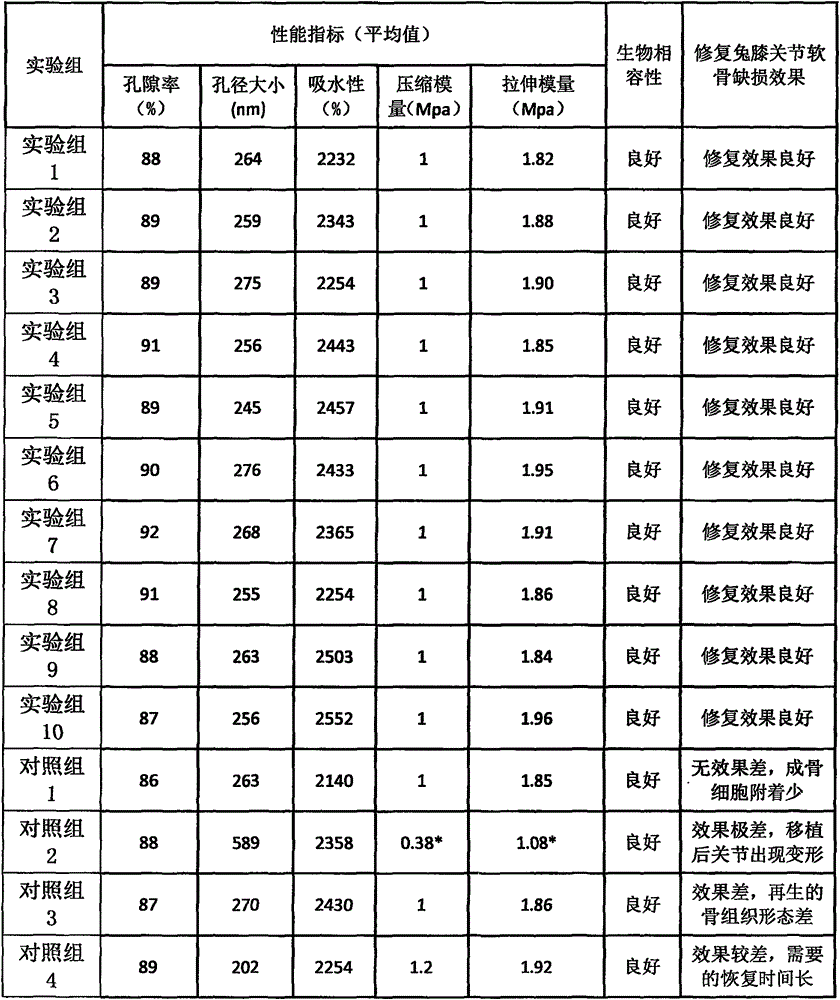
Bone induction material and preparation method and application thereof
InactiveCN101249278AImprove biological activitySustained-release trace high-efficiencyPeptide/protein ingredientsSkeletal disorderFiberOSTEOINDUCTIVE FACTOR
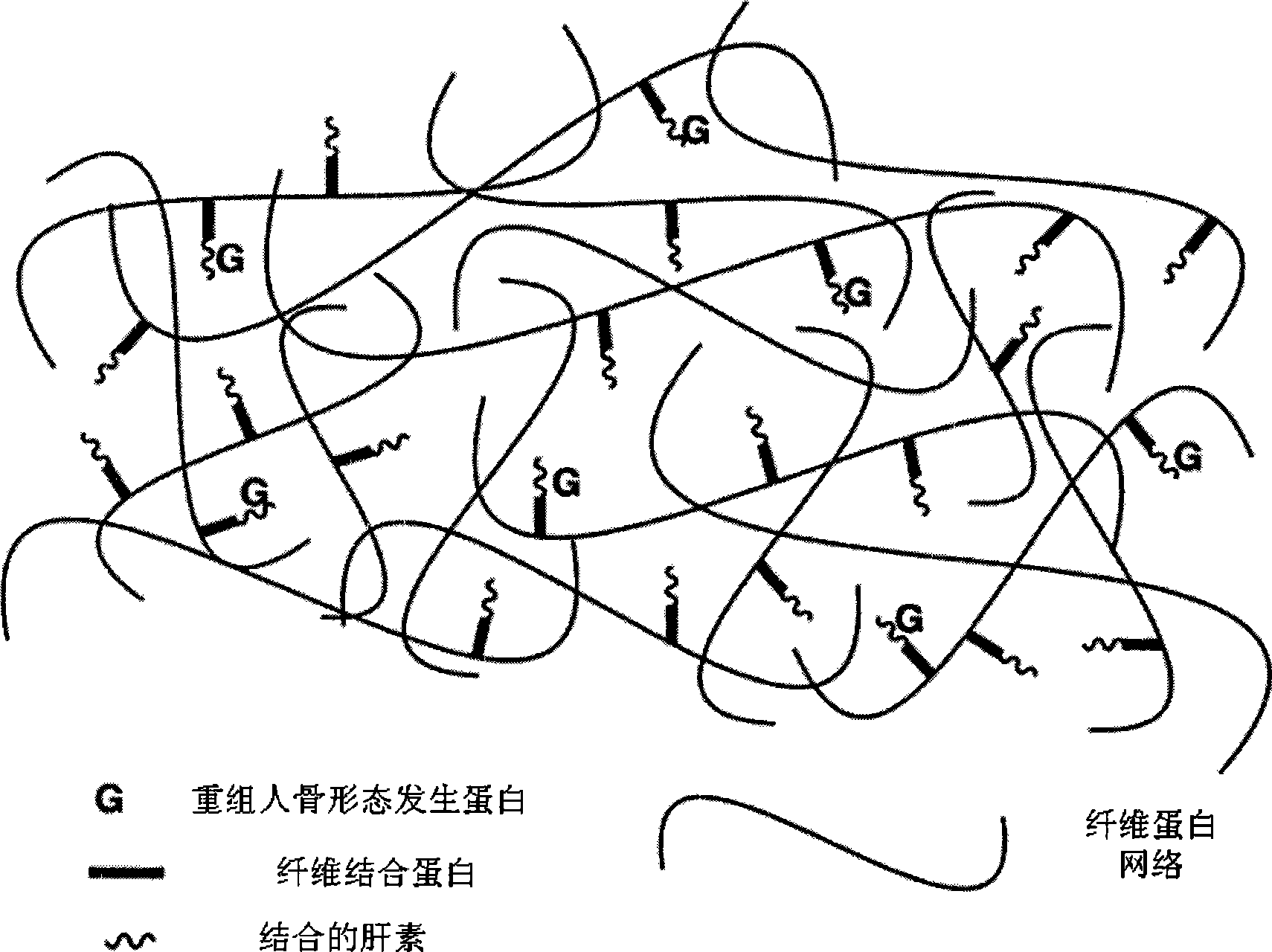
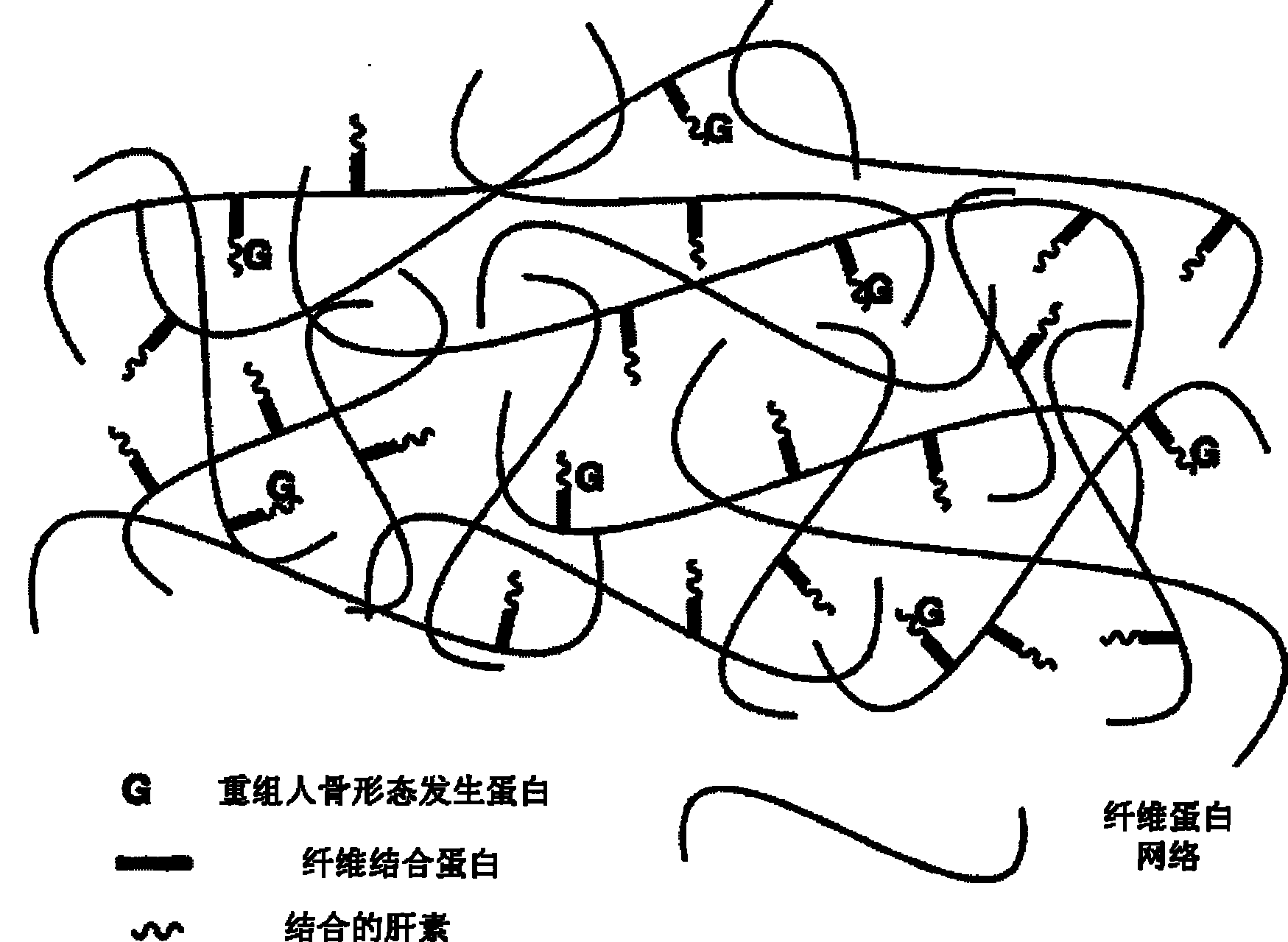
The invention discloses a bone induction material which comprises bone induction factors and carriers; the bone induction factors comprise bone morphogenetic proteinbmp or basic fiberblast growth factors, and the carriers form gel matrix through the interaction among fibernogen, fibernectin, heparin, fiber protein stabling factor, thrombin and calcium chloride; the bone induction factor is embedded in the gel matrix. The invention further discloses a preparation method and application of bone induction material, which can release the bone morphogenetic proteinbmp slightly, efficiently and stably, and can improve the biological activity of bone morphogenetic proteinbmp.
Owner:RESEARCH INSTITUTE OF TSINGHUA UNIVERSITY IN SHENZHEN
Implants comprising an osteoinductive factor and a contrast agent compatible therewith
The present invention is directed to methods, systems and reagents for providing contrast media that are compatible with osteoinductive factor induced bone formation.
Owner:WARSAW ORTHOPEDIC INC
Devices and methods for treating defects in the tissue of a living being
InactiveUS9981061B2Restore mechanical and architectural and structural competenceProvide integrityInternal osteosythesisPharmaceutical delivery mechanismMedicineOSTEOINDUCTIVE FACTOR
Owner:DSM IP ASSETS BV
Tissue interface augmentation device for ligament/tendon reconstruction
ActiveUS10080644B2Promote reconstructionPromote osseointegrationLigamentsMusclesFiberOSTEOINDUCTIVE FACTOR
The present invention relates to a device for the interfacial augmentation of tissue grafts for the purpose of tendon and ligament reconstruction. The inventive device works both as a delivery vessel for osteoconductive and osteoinductive factors and as a scaffold to stimulate and support bone ingrowth and comprises a tubular composite silk sheath, said tubular composite silk sheath comprising: a backbone consisting of a tubular silk mesh, and a carrier material consisting of a porous silk sponge, wherein said tubular silk mesh consists of degummed silk fibroin fibers, wherein said porous silk sponge comprises silk fibroin fibers and hydroxyapatite particles and said tubular silk mesh and said porous silk sponge form a composite material. The present invention is also directed to a method for the manufacturing of such augmentation devices, a method for fixation of the thus fabricated tissue interface augmentation devices onto ligament or tendon grafts, a method for applying such devices for ligament and / or tendon reconstruction to tendon grafts, as well as their application in ligament and tendon reconstruction.
Owner:NAT UNIV OF SINGAPORE +1
Methods, systems, and reagents for delivery to bone of an osteoinductive factor with a contrast agent
InactiveUS20070202048A1Improve bone formationNanotechPeptide/protein ingredientsOSTEOINDUCTIVE FACTORContrast medium
The present invention is directed to methods, systems and reagents for providing contrast media that are compatible with osteoinductive factor induced bone formation.
Owner:WARSAW ORTHOPEDIC INC
Preparation method of dextral hydrogel material
InactiveCN109337864AControl fateSolve the technical difficulty of precisely regulating the adipogenic differentiation of stem cellsSkeletal/connective tissue cellsCell culture supports/coatingFiberOSTEOINDUCTIVE FACTOR
The invention relates to a preparation method of a dextral hydrogel material, and solves the technical problem that an existing matrix material can hardly precisely regulate adipogenic differentiationof stem cells. The preparation method comprises mainly the following main steps: (1) dissolving a dextral gel factor in a dimethyl sulfoxide solution to obtain a dextral gel factor solution with a volumetric concentration of 12 to 33 mg / [mu]l, and placing the solution at the bottom of a 24-pore plate; (2) uniformly mixing the prepared dextral gel factor solution with a culture medium suspension of mesenchymal stem cells in the 24-pore plate, and standing still at 30 to 40 DEG C for 30 to 60 minutes to form dextral hydrogel; (3) putting the prepared dextral hydrogel into an osteoinductive factor-free culture medium suspension of mesenchymal stem cells for culturing, and replacing the culture medium of the mesenchymal stem cells on the dextral hydrogel at intervals. The preparation method can be widely applied to the field of hydrogel fibers for regulating and controlling the adipogenic differentiation of the three-dimensional mesenchymal stem cells.
Owner:PEKING UNIV SCHOOL OF STOMATOLOGY +1
Spinal fusion methods and devices
ActiveUS8226729B2Minimizing undesirable migrationReduce formationBone implantSkeletal disorderPosterior approachOSTEOINDUCTIVE FACTOR
Owner:WARSAW ORTHOPEDIC INC
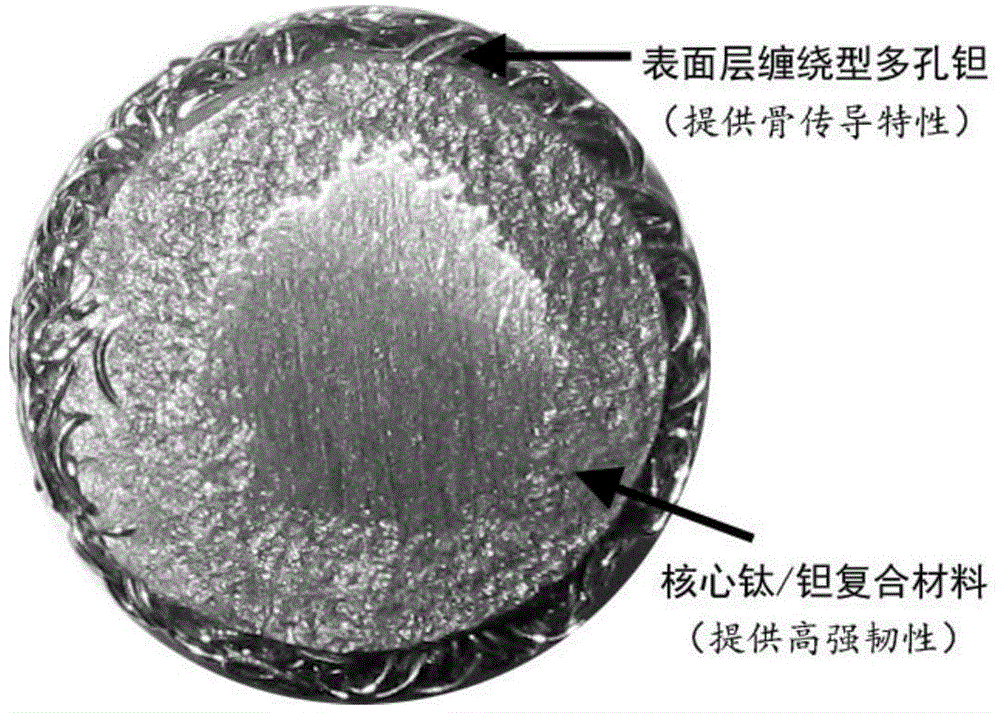
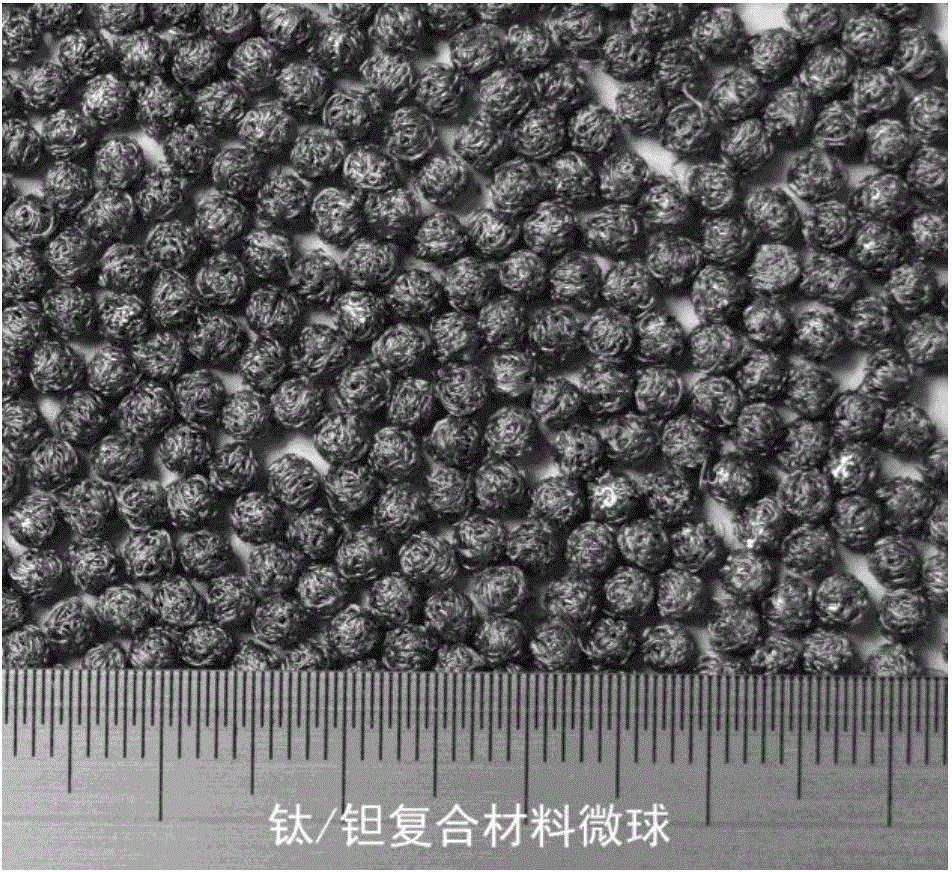
Titanium and tantalum composite microsphere bone filling medical material
A titanium and tantalum composite microsphere bone filling medical material is characterized in that the bone filling medical material is a titanium and tantalum composite microsphere, the titanium and tantalum composite microsphere comprises a microsphere core made of a titanium / tantalum two-phase composite material and a microsphere surface formed by a winding tantalum porous layer, the titanium / tantalum two-phase composite material is formed through the steps of preparing a porous microsphere by braiding medical tantalum or tantalum alloy wires, injecting a liquid titanium or titanium alloy melt into the center of the porous microsphere, cooling and solidifying, and the microsphere surface includes a space for surface bioactive coating and medicine loading. The titanium and tantalum composite microsphere bone filling medical material substantially improves the strength and rigidity of the bone filling medical material, realizes high fatigue life in biological environment, maintains excellent biocompatibility and bone conduction, provides enough space and possibility for medicine loading and loading of bone inducement factors, and is suitable for various bone filling, restoration, reconstruction and other orthopedic clinic applications under minimal invasion conditions.
Owner:SHANGHAI JIAO TONG UNIV
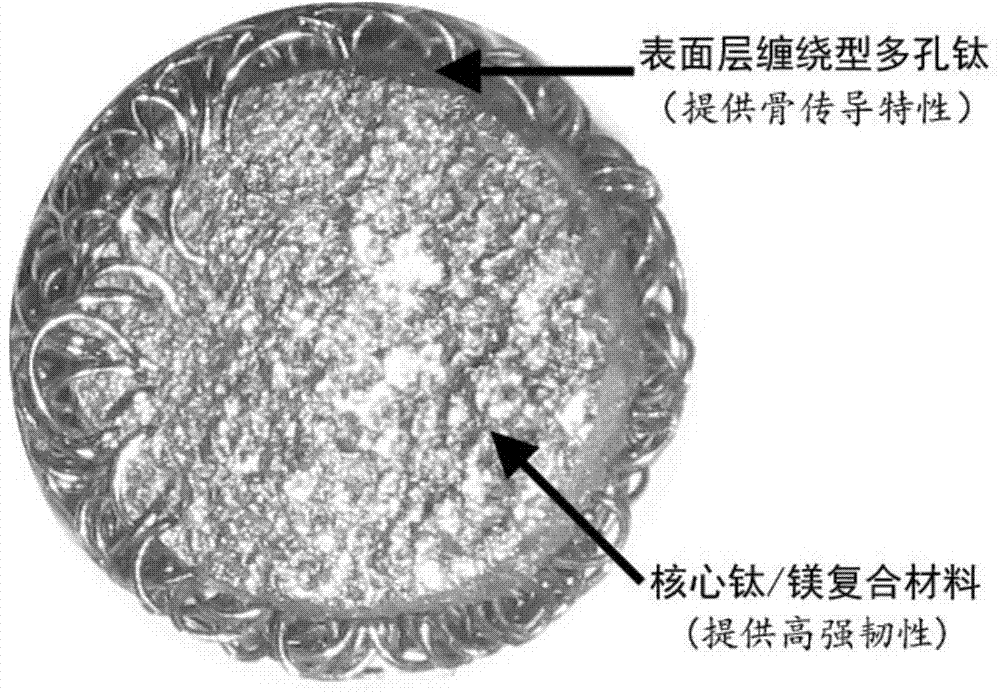
Medical semi-degraded titanium-magnesium composite microsphere bone filling material
InactiveCN104707169AHigh strengthIncrease stiffnessProsthesisBiocompatibility TestingOSTEOINDUCTIVE FACTOR
The invention discloses a medical semi-degraded titanium-magnesium composite microsphere bone filling material which comprises titanium-magnesium composite microspheres. The titanium-magnesium composite microspheres are woven into porous microspheres by medical titanium or titanium alloy wires, wherein liquid magnesium or magnesium alloy melt liquid is injected into the centers of the porous microspheres; the titanium / magnesium two-phase composite material formed after cooling and solidifying are used for preparing microsphere cores and microsphere surfaces formed by winding type titanium porous layers; spaces for biological activity coating of the surfaces and loading of drug are formed in the surfaces of the microspheres; the magnesium in the titanium / magnesium two-phase composite material is degraded in the biological environment; the degradation rate can be regulated. By virtue of the medical semi-degraded titanium-magnesium composite microsphere bone filling material, the strength and stiffness of the bone filling material are improved; the fatigue life of the biological environment is prolonged; the excellent biocompatibility and osteoconduction are retained; the long-term stability of the implant in the biological environment can be retained by degradation of magnesium and growth of new bone; the space and the possibility of loading drugs and loading bone induction factors are also provided; the material is suitable for orthopedic clinical application for filling, repairing and rebuilding various bones in the minimally-invasive situation.
Owner:SHANGHAI JIAO TONG UNIV
Spinal fusion methods and devices
InactiveUS20090298776A1Minimizing undesirable migrationReduce formationPeptide/protein ingredientsBone implantPosterior approachOSTEOINDUCTIVE FACTOR
Methods, devices and compositions for fusing adjacent vertebrae, and otherwise localizing bone growth, are provided. In one form of the invention, a method for fusing adjacent vertebrae includes preparing a disc space for receipt of an intervertebral disc implant in an interwertebral disc space between adjacent vertebrae, inserting the implant into the intervertebral disc space and providing an osteoinductive composition that includes an osteoinductive factor in a pharmaceutically acceptable carrier. The carrier is advantageously substantially impermeable to efflux of the osteoinductive factor and is released as the carrier is resorbed or biodegraded. Preferred carriers include a hardened, resorbable carrier, such as a calcium phosphate cement that retains at least about 50% of the osteoinductive factors greater than about 2 days. Preferred osteoinductive factors are growth factors and include bone morphogenctic proteins and LIM mineralization proteins. In alternative forms of the invention, the method may be performed without utilization of a load-bearing spinal implant by disposing the osteoinductive composition in the disc space. The method is advantageously performed on lumbar vertebrae by a posterior approach. Intervertebral fusion devices and methods for their preparation are also provided.
Owner:WARSAW ORTHOPEDIC INC
Devices and methods for treating defects in the tissue of a living being
InactiveUS20170266342A1Provide structural integrityRestore mechanical and architectural and structural competenceInternal osteosythesisPharmaceutical delivery mechanismOSTEOINDUCTIVE FACTORCeramic
An implant for deployment in select locations or select tissue for regeneration of tissue is disclosed. The implant includes collagen and or other bio-resorbable materials, where the implant may also be used for therapy delivery. Additionally, the implant may include, or have blended in, an additive, such as an osteoinductive factor, for example biocompatible ceramics and glass.
Owner:DSM IP ASSETS BV
Biological gel implant for maxillary sinus and preparation method of biological gel implant
InactiveCN104274250AIncrease the areaIncrease in sizeDental implantsDental prostheticsNumerical controlRelease time
The invention relates to a biological gel implant for maxillary sinus and a preparation method of the biological gel implant. A porous surface structure and an internal hollow structure are manufactured by adopting a 3D printing technology combined with a CNC (Computer Numerical Control) method, wherein a lot of biological gel with osteoinductive factors is stored in the porous surface structure and the internal hollow structure; the biological gel implant comprises an implant section, a drill base, a center screw, biological gel and an aseptic packaging bottle; the top end of the implant section is connected with the drill base by virtue of screws; an outside cap is arranged at the top of the implant section; the neck of the implant section is provided with micro threads; the lower part of the implant section is of a porous surface structure and an internal hollow structure; a texture surface is formed in the hole wall; the porous structure is communicated with the internal hollow structure; a lot of biological gel with osteoinductive factors can be stored; and the concentration and release time of the biological gel are much superior to those of a biological coating implant at present. The biological gel implant has a skeleton-like hollow structure capable of inducing bone tissues to actively grow into the implant, the new bone fusion area and bone-formation strength of the implant can be greatly increased, and the biological gel implant is suitable for a maxillary sinus implant surgery under the condition of osteoporosis.
Owner:南京星洁医疗科技有限公司
Viral and non-viral vectors as vehicles for delivering transgenes for treating bone pathologies
InactiveUS20110280935A1Genetic material ingredientsSkeletal disorderDelivery vehicleOSTEOINDUCTIVE FACTOR
The present invention relates to a method for treating bone pathologies comprising delivering a viral or non-viral delivery vehicle comprising genetic information (e.g. a transgene) encoding a therapeutic osteoinductive factor to target cells in vivo enabling the cells to produce the osteoinductive factor at the site of the bone pathology. The delivery is achieved by a simplified method which does not require cumbersome ex vivo techniques or additional matrix or scaffolding agents. Such viral and non-viral delivery vehicles of the present invention are derived from the following nonlimiting examples: adenoviruses, adeno-associated viruses, retroviruses, herpes simplex viruses, liposomes, and plasmids. The osteoinductive factors include, but are not limited to, growth factors, cytokines, growth factor inhibitors and cytokine inhibitors.
Owner:BALTZER AXEL W +2
Devices and methods for treating defects in the tissue of a living being
InactiveUS20180078675A9Restore mechanical and architectural and structural competenceProvide integrityInternal osteosythesisPharmaceutical delivery mechanismMedicineOSTEOINDUCTIVE FACTOR
An implant for deployment in select locations or select tissue for regeneration of tissue is disclosed. The implant includes collagen and or other bio-resorbable materials, where the implant may also be used for therapy delivery. Additionally, the implant may include, or have blended in, an additive, such as an osteoinductive factor, for example biocompatible ceramics and glass.
Owner:DSM IP ASSETS BV
Bone induction material and preparation method and application thereof
InactiveCN101249278BImprove biological activitySustained-release trace high-efficiencyPeptide/protein ingredientsSkeletal disorderFiberOSTEOINDUCTIVE FACTOR
The invention discloses a bone induction material which comprises bone induction factors and carriers; the bone induction factors comprise bone morphogenetic proteinbmp or basic fiberblast growth factors, and the carriers form gel matrix through the interaction among fibernogen, fibernectin, heparin, fiber protein stabling factor, thrombin and calcium chloride; the bone induction factor is embedded in the gel matrix. The invention further discloses a preparation method and application of bone induction material, which can release the bone morphogenetic proteinbmp slightly, efficiently and stably, and can improve the biological activity of bone morphogenetic proteinbmp.
Owner:RESEARCH INSTITUTE OF TSINGHUA UNIVERSITY IN SHENZHEN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com