Bone induction material and preparation method and application thereof
An osteoinductive and factor-based technology, applied in bone diseases, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problems of not being able to protect BMPs from inhibition and degradation, and not being able to effectively improve the biological activity of BMPs, so as to achieve protection from degradation , enhance biological activity, improve the effect of biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
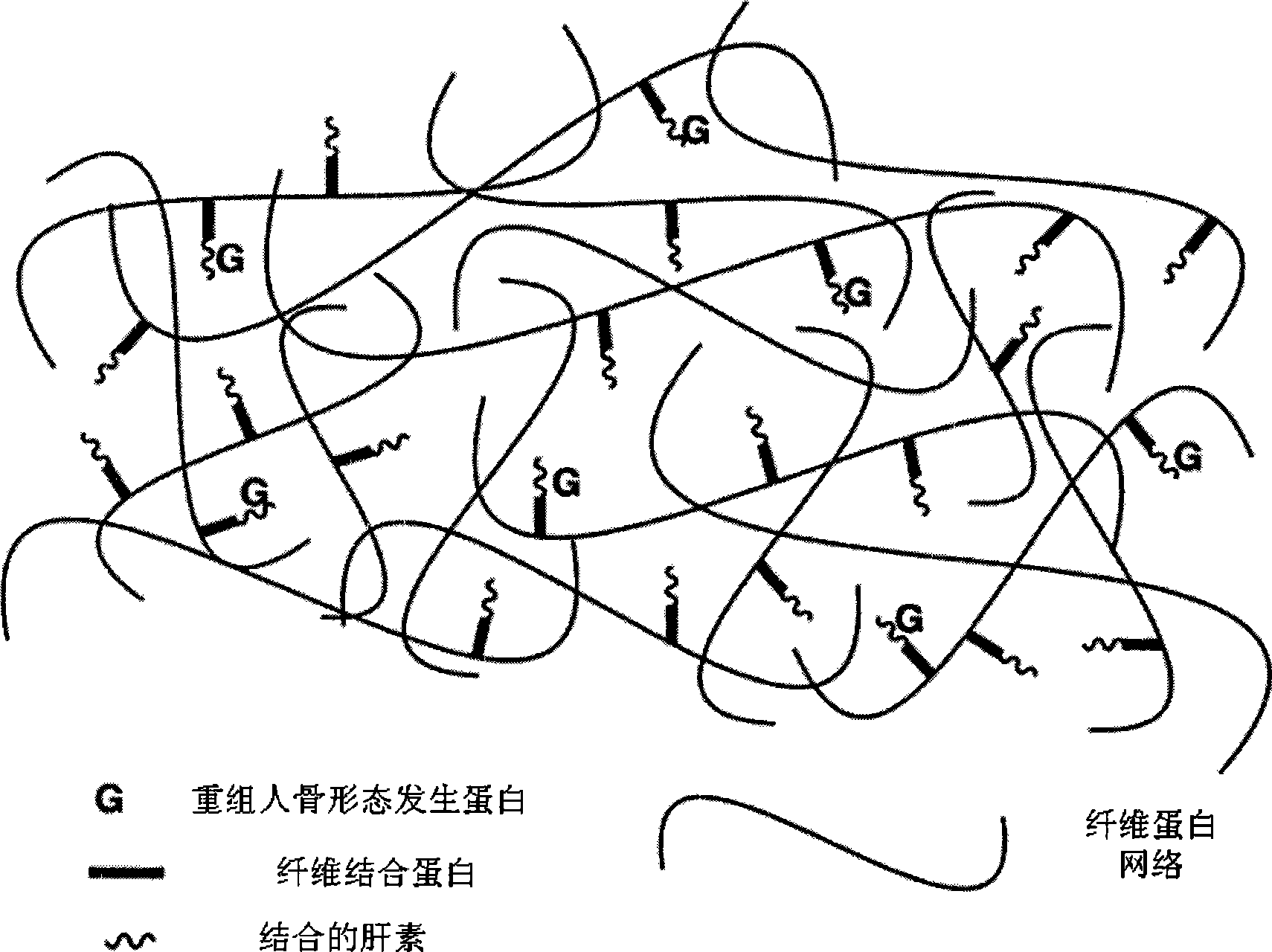
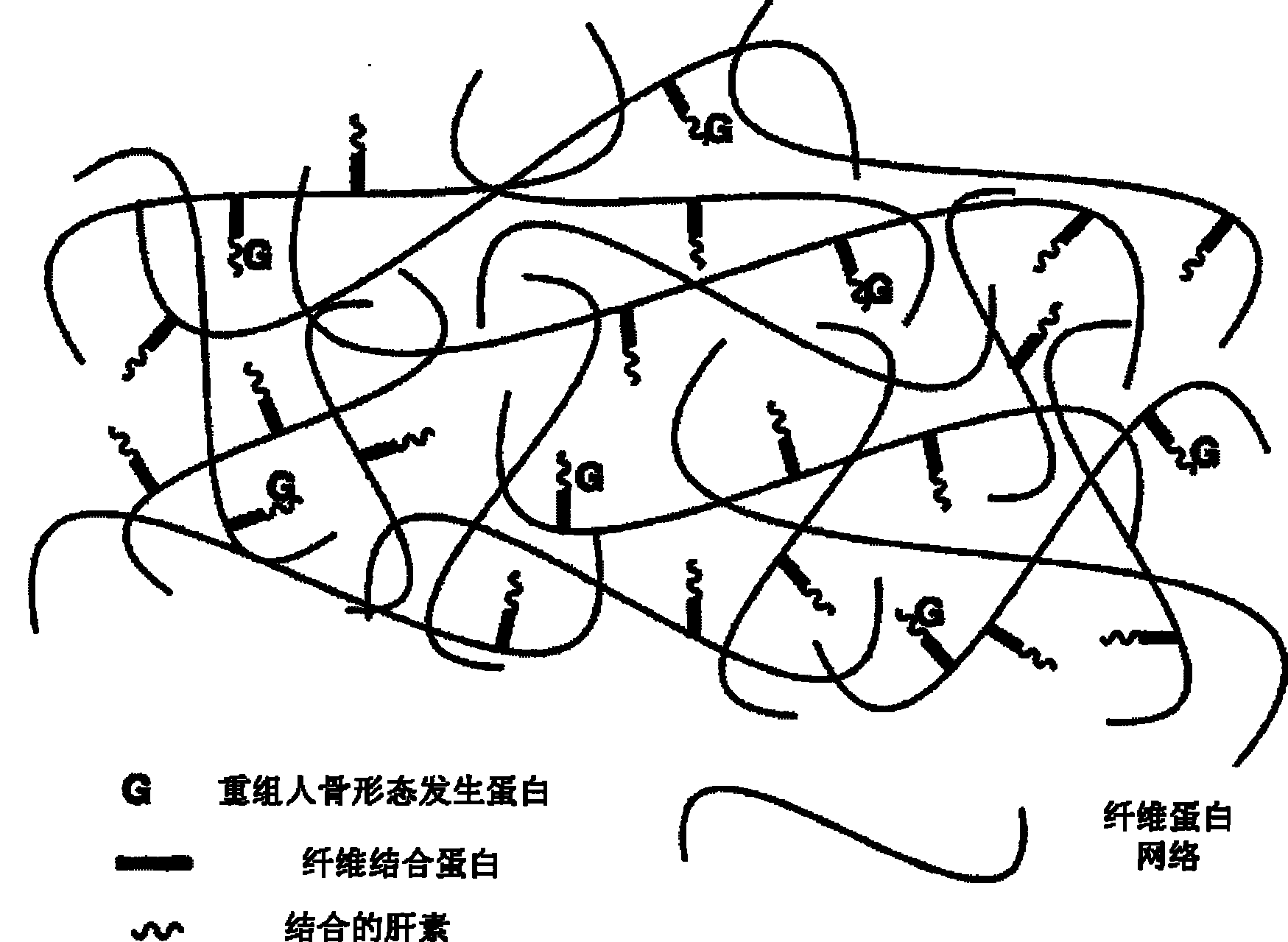
[0034] The rhBMP-2 sustained-release system is temporarily prepared at the time of application, and is formed by mixing the following three components in sequence. Component I is rhBMP-2 lyophilized powder; Component II is a solution containing fibrinogen, fibronectin, heparin and factor XIII, pH 9.0, wherein the concentration of fibrinogen in Component II is 120mg / ml, the molar ratio of fibrinogen to fibrinogen is 10:1, the molar ratio of fibrinogen to heparin is 10:1, in component II, every 1mg of fibrinogen corresponds to adding 1.2IU of Factor XIII; Component III is a solution containing thrombin and calcium chloride, pH9.0, the concentration of said thrombin in component III is 800IU / ml, said CaCl 2 The concentration in fraction III is 45umol / ml. During preparation, add component II to component I, and put it in a water bath at 37°C for 15 minutes to dissolve the rhBMP-2 lyophilized powder. The mass ratio of rhBMP-2 to heparin is 1:50, and then Add component III, shake...
Embodiment 2
[0036] The rhBMP-2 sustained-release system is temporarily prepared at the time of application, and is formed by mixing the following three components in sequence. Component I is rhBMP-2 freeze-dried powder; Component II is a solution containing fibrinogen, fibronectin, heparin and factor XIII, pH 8.5, wherein the concentration of fibrinogen in Component II is 60mg / ml, the molar ratio of fibrinogen to fibrinogen is 5: 1, the molar ratio of fibrinogen to heparin is 5: 1, in component II, every 1mg of fibrinogen corresponds to adding 0.65IU of Factor XIII; component III is a solution containing thrombin and calcium chloride, pH8.6, the concentration of said thrombin in component III is 450IU / ml, said CaCl 2 The concentration in fraction III is 40umol / ml. During preparation, add component II to component I, and place it in a water bath at 35°C for 25 minutes to dissolve the rhBMP-2 lyophilized powder. The mass ratio of rhBMP-2 to heparin is 1:25, and then Add component III, sh...
Embodiment 3
[0038] The rhBMP-2 sustained-release system is temporarily prepared at the time of application, and is formed by mixing the following three components in sequence. Component I is rhBMP-2 lyophilized powder; Component II is a solution containing fibrinogen, fibronectin, heparin and factor XIII, pH6.8, wherein the concentration of fibrinogen in Component II is 4mg / ml, the molar ratio of fibrinogen to fibrinogen is 1:1, the molar ratio of fibrinogen to heparin is 1:1, in component II, every 1mg of fibrinogen corresponds to adding 0.3IU of Factor XIII; Component III is a solution containing thrombin and calcium chloride, pH6.9, the concentration of said thrombin in component III is 40IU / ml, said CaCl 2 The concentration in fraction III is 35umol / ml. During preparation, add component II to component I, and dissolve in a water bath at 33°C for 30 minutes to fully dissolve the rhBMP-2 lyophilized powder. The mass ratio of rhBMP-2 to heparin is 1:1, and then Add component III, shak...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com