Spinal fusion methods and devices
a spine and fusion technology, applied in the field of spine fusion methods and devices, can solve the problems of back pain, rupture or degeneration of intervertebral discs, and middle-aged working population's disability, and achieve the effects of reducing bone formation, promoting and limiting bone growth, and minimizing undesirable migration of osteoinductive composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
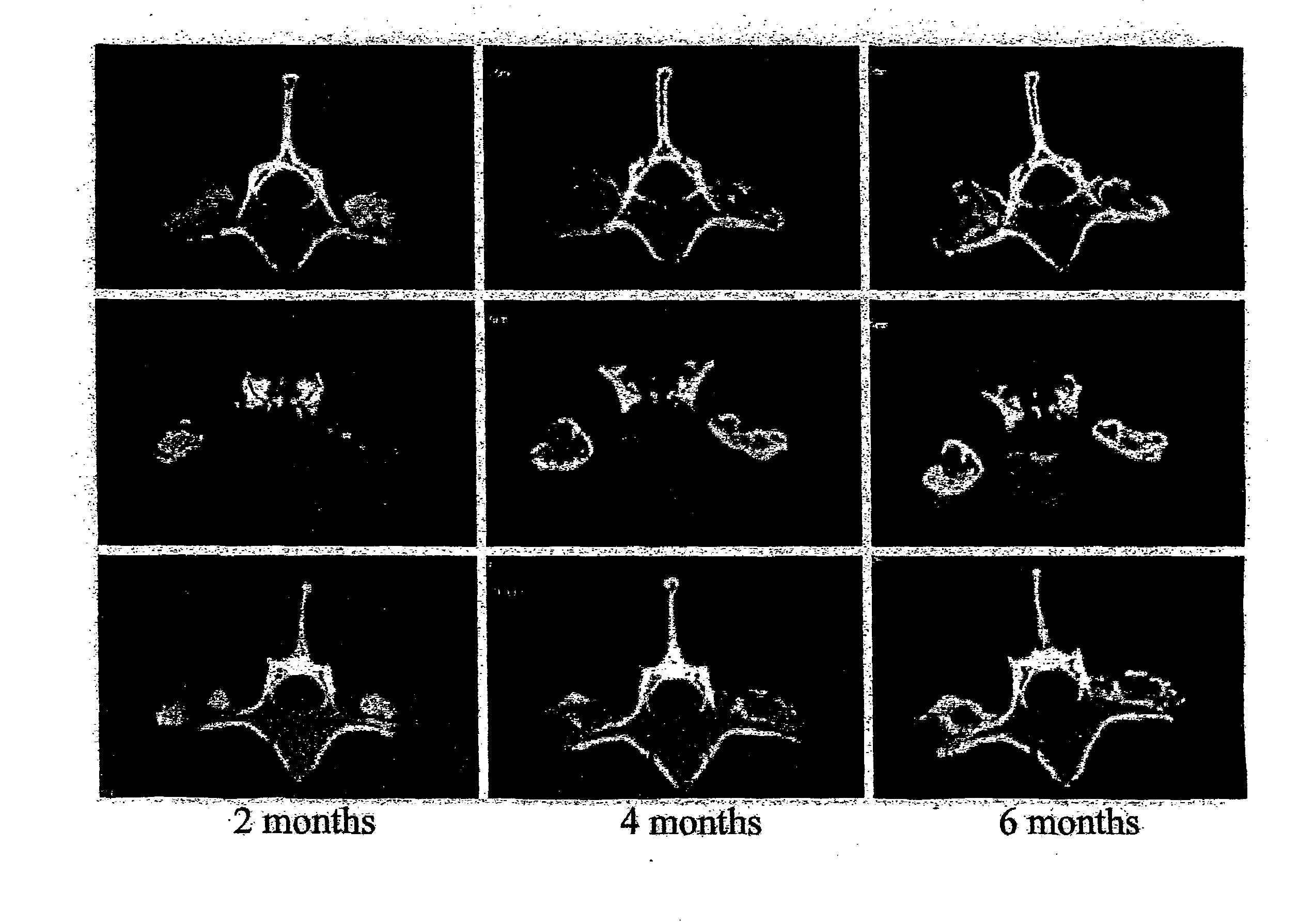
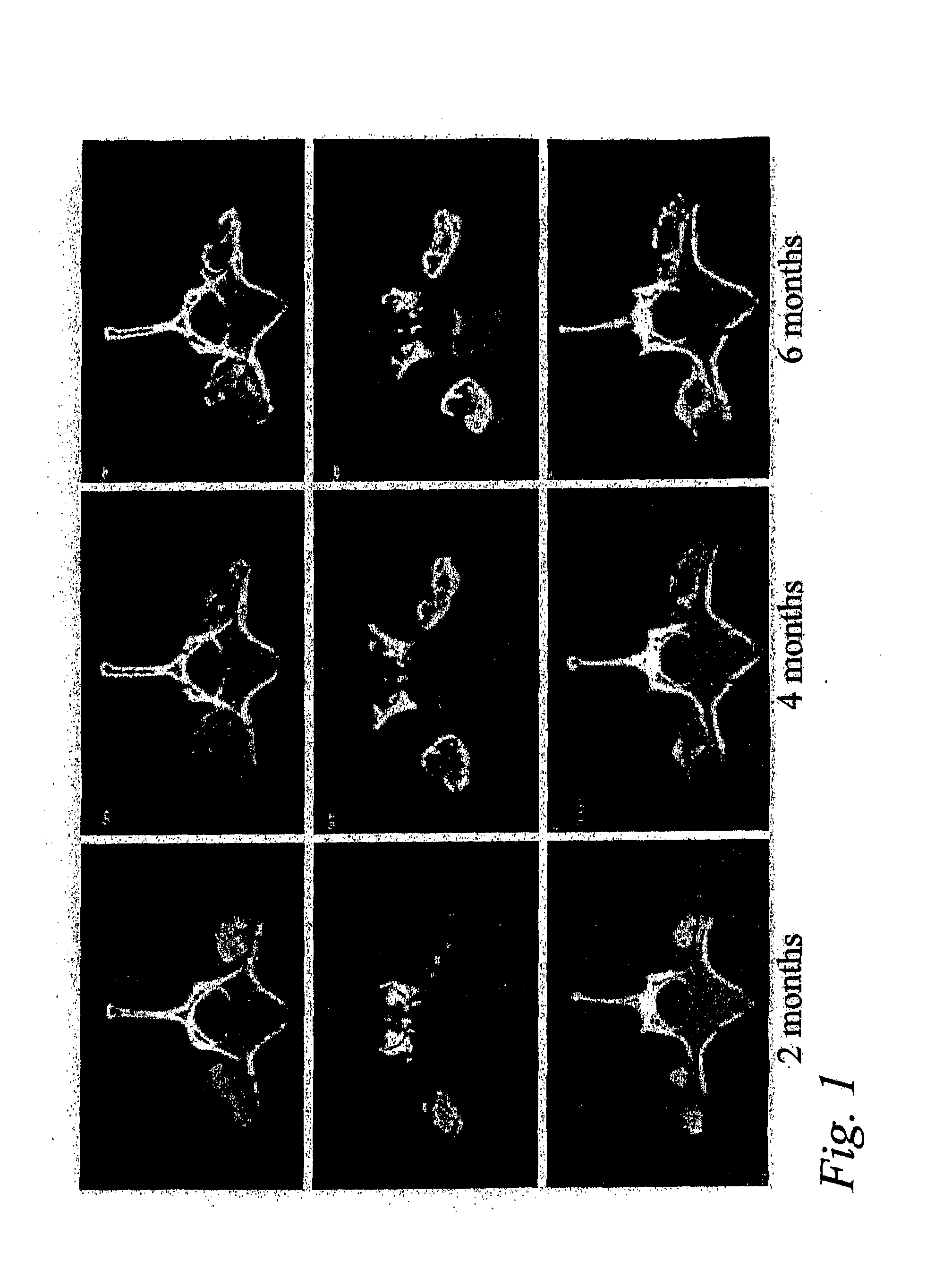
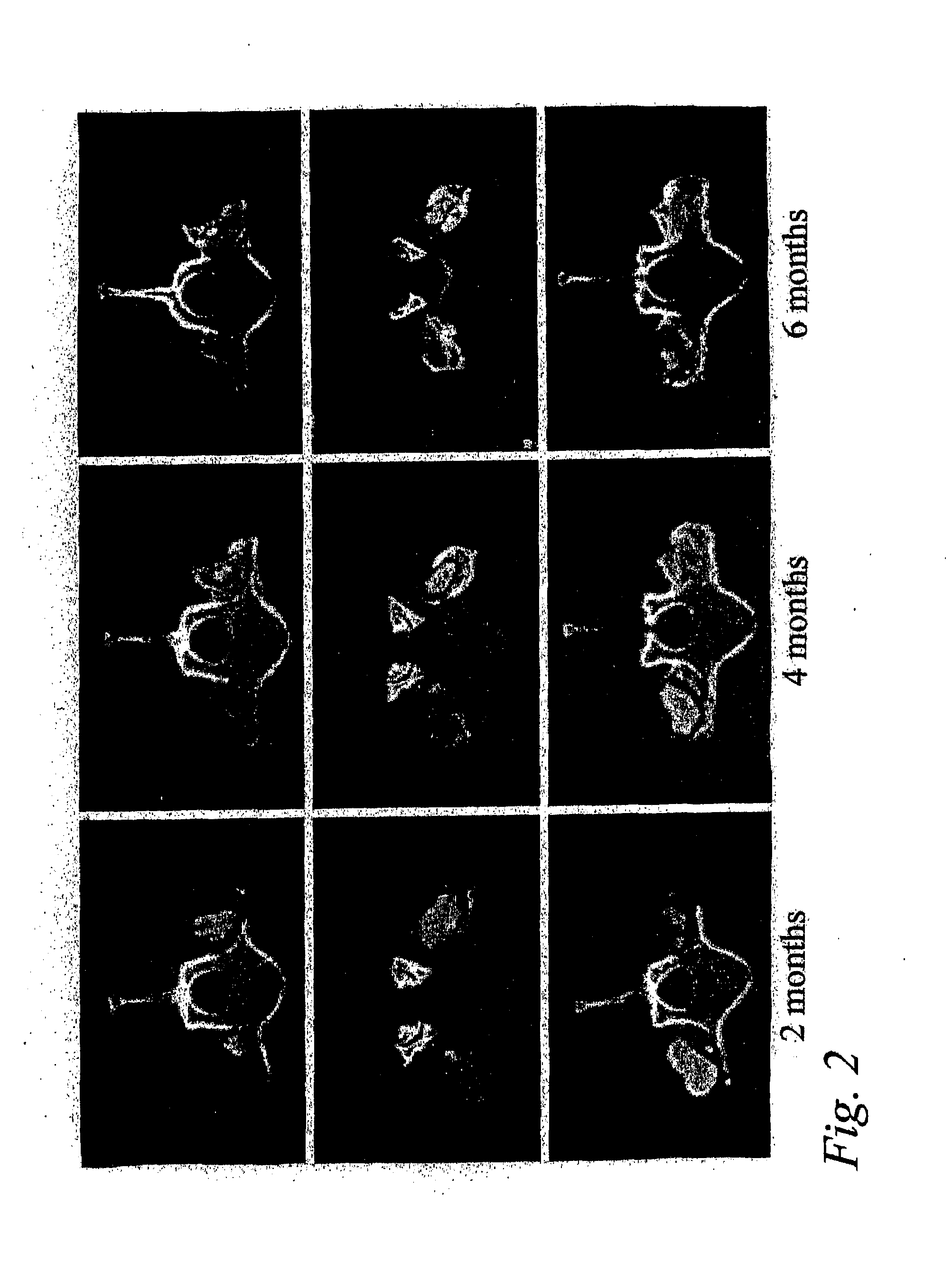
Single-level Posterolateral Fusion in Rhesus Monkeys
[0048]This example shows that posterolateral fusions performed in Rhesus monkeys with the bone substitute compositions described herein resulted in new bone formation that was confined to the volume occupied by the bone substitute compositions.
Animals and Experimental Design
[0049]Posterolateral transverse process fusions in 2 groups of 2 Rhesus monkeys were performed. One of the groups received rhBMP-2 in a carrier of standard α-bone substitute material (standard α-BSM®), a commercially available calcium phosphate cement purchased from Etex Corp., Cambridge, Mass. This standard material has a microporosity of 40%. The other group received rhBMP-2 in a carrier of modified α-BSM® (a bone substitute material from Etex with a porosity greater than 40%). In this second group, the left side of the spine was treated with the rhBMP-2 in a carrier of Δ-BSM® having 80% porosity (the porosity was increased by increasing the liquid content) an...
example 2
Pharmacokinetic Study of the Release of rhBMP-2 From α-BSM and ACS
[0053]The release kinetics for rHBMP-2 from α-BSM and ACS evaluated in a rabbit ulna osteotomy. A 125I-rhBMP-2 / α-BSM or 125IrhBMP-2 / ACS product was surgically implanted in a rabbit ulna osteotomy. Assessment of the radioactivity at the implant site were made as soon as possible following surgery. Additionally assessments were made periodically thereafter including at 1, 2, 3, 4, 7, 14 and 21 days after surgery.
[0054]The rhBMP-2 was radiolabeled with 125I using the iodogen technique. The following a typical procedure. An 80 μg / mL solution of iodogen reagent (Pierce, Rockford, Ill.) was prepared in chloroform. An aliquot of this solution (50 μL) was placed into a micro-eppendorf tube and evaporated to dryness under a gentle stream of nitrogen. To this tube was added 30 μg of rhBMP-2, sufficient MFR 00842 buffer to bring the volume up to 50 μL, and 1-2 mCi of carrier-free 125I (Dupont NEN Research Product, Boston Mass.)....
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com