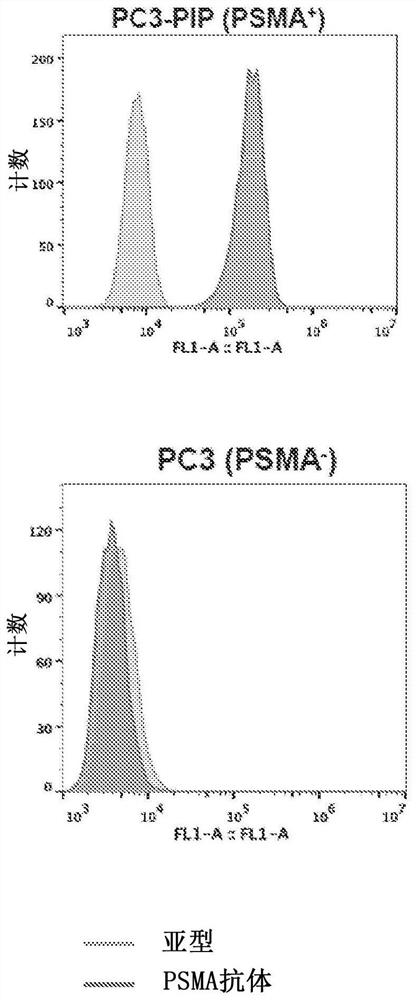
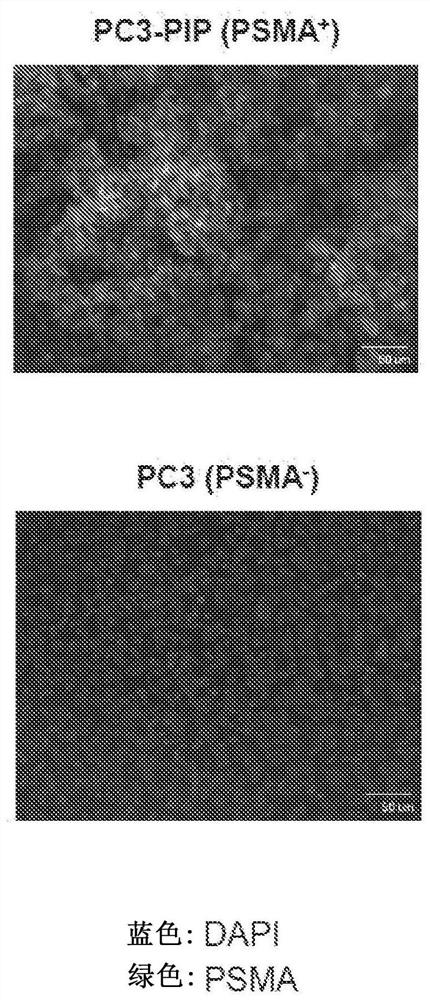
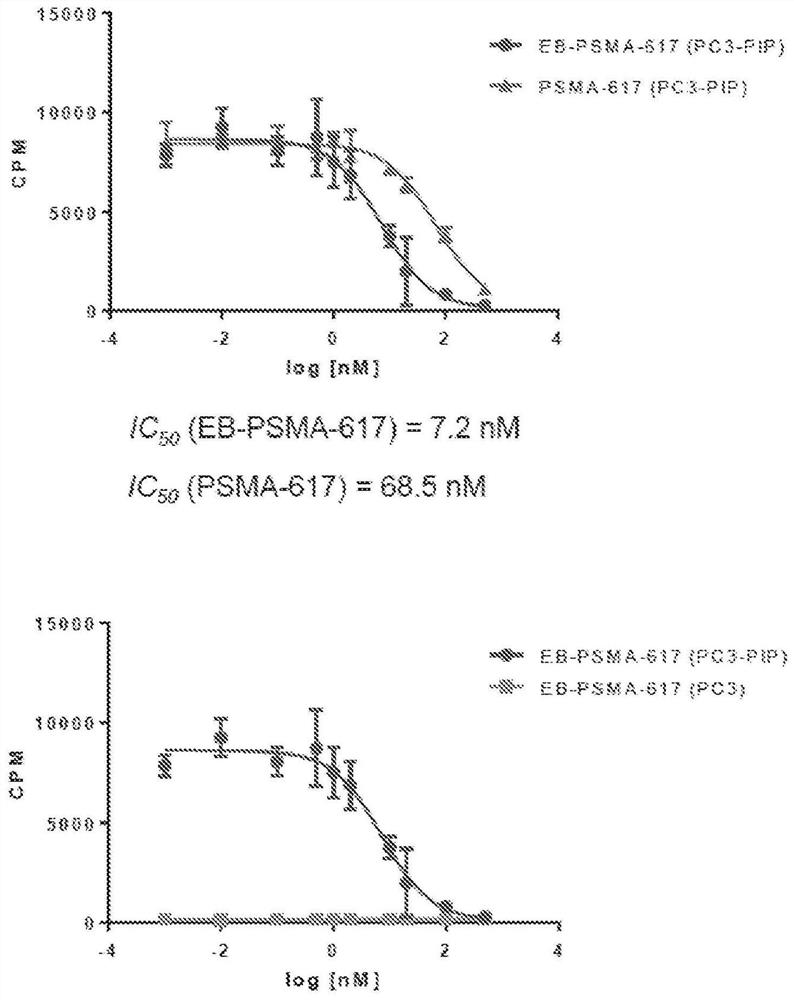
Chemical conjugates of evans blue derivatives and their use as radiotherapy and imaging agents for targeting prostate cancer
A technology of compounds, radionuclides, applied in the field of functionalized derivatives of Evans blue dye
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0095] One aspect of the present invention includes a chemical conjugate of Evans blue dye having a compound of formula I shown below, or a pharmaceutically acceptable ester, amide, solvate or salt thereof, or a salt of such an ester or amide or solvates of such ester amides or salts:
[0096]
[0097] In formula I, the substituent R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R 9 , R 10 and R 11 independently selected from hydrogen, halogen, hydroxyl, cyano, C 1 -C 6 Alkyl, C 1 -C 6 Alkoxy, C 1 -C 6 Haloalkyl and C 1 -C 6 Haloalkoxy. R 12 for hydrogen, C 1 -C 6 Alkyl or C 1 -C 6 Haloalkyl. R 13is a chelating group. R 14 is a group capable of binding prostate-specific membrane antigen (PSMA).
[0098] Formula I may also include a linking group L 1 , which is -(CH 2 ) m -, wherein m is an integer from 0 to 12; linking group L 2 Yes-(CH 2 ) n -, wherein n is an integer from 0 to 12; and the linking group L 3 Yes-(CH 2 ) p -, wherein p is...
Embodiment 1
[0181] Embodiment 1: the synthesis of DOTA-maleimide-EB (DMEB)
[0182] Step 1-2: Synthesis of benzidine-Lys-Boc
[0183]
[0184] To a 100 mL round bottom flask containing 1.1 g of Boc-lysine-Fmoc amino acid (1 eq) and anhydrous N,N-dimethylformamide (DMF) (10 mL) was added HATU (0.94 g ,1.05eq). The mixture was stirred at room temperature (RT) for 10-15 minutes. Then, diisopropylethylamine (DIPEA) (4 mL, 10 eq) was added, followed by a solution of benzidine (0.75 g, 1.5 eq) in 10 mL of DMF. The reaction mixture was stirred overnight. Conversion to the target product was evaluated by analytical HPLC using System 1 (retention time 32.3 minutes). Solvent was removed by rotary evaporator using an oil vacuum pump. The remaining oil was redissolved in 5-10 mL of DMF, followed by the addition of 20% piperidine (v / v). The mixture was stirred at room temperature for 15-20 and assessed for deprotection of Fmoc by injection into analytical HPLC system 1 (retention time 18.7 mi...
Embodiment 2
[0198] Example 2: Alternative Synthesis of DOTA-Maleimide-EB (DMEB)
[0199] Step 1: Evansblue amine (EB-NH 2 ) preparation
[0200] To a 100ml round bottom flask containing 2-toluidine (4.3g) and dichloromethane (40ml) was added di-tert-butyl dicarbonate (4.4g). The mixture was stirred overnight at room temperature. The reaction was concentrated, and the residue was purified by silica gel chromatography to obtain 3.2 g of N-Boc-2-benzylidine. LC-MS: [MH]+ = 313.4135 (m / z), Calculated: 312.1838.
[0201]
[0202] In a glass vial, N-Boc-2-toluidine (0.46g, 1.47mmol) was dissolved in acetonitrile (10ml), cooled to 0°C, then hydrochloric acid (0.3M, 15ml) was added. Cold sodium nitrite solution (0.31 g in 5 ml of water) was added dropwise and stirred for 20 minutes, the solution turned bright yellow. At 0°C, this solution was added dropwise to a solution of 1-amino-8-naphthol-2,4-disulfonic acid monosodium salt (0.59 g) and sodium bicarbonate (0.49 g) in water (20 ml). i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com