A kind of polypeptide prodrug modified by Evans blue and its preparation and application
A technology of Evans Blue and peptides, which is applied to medical preparations with no active ingredients, medical preparations containing active ingredients, peptides, etc., can solve the problems of low curative effect, large side effects, high price, etc., and achieve stable blood circulation , reduce toxic side effects, strong cytotoxic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1: the preparation of EB-VcMMAE
[0069] Synthesize EB-SH according to the following synthetic route:
[0070]
[0071] Specific steps include:
[0072] Drop into 3,3'-dimethylbenzidine (4.18g, 2eq.) and N-Boc-γ-aminobutyric acid (2g, 1eq) respectively in a 500mL flask containing 250 acetonitrile, and add 2-(7 -benzotriazole oxide)-N,N,N',N'-tetramethyluronium hexafluorophosphate (HATU, 7.47g, 2eq) and N,N-diisopropylethylamine (DIPEA, 6.3g, 5eq). Under stirring at room temperature, after 24 hours of reaction, the solvent was spin-dried, and the residue was purified through a silica gel column to obtain the brown intermediate BT. In an ice bath, 15 mL of ice-cold 2.0M HCl was added dropwise to 40 mL of BT (3.98 g, 10.0 mmol) in acetonitrile. Stir for 15min, then add 20mL NaNO 2 (2.07g, 30.0mmol) ice-water solution was slowly added dropwise to the flask, after the dropwise stirring was continued for 30min to generate a yellow diazonium salt solution, w...
Embodiment 2
[0074] Embodiment 2: Preparation of EB1-VcMMAE
[0075]Synthesize EB-SH according to the following synthetic route:
[0076]
[0077] Specific steps include:
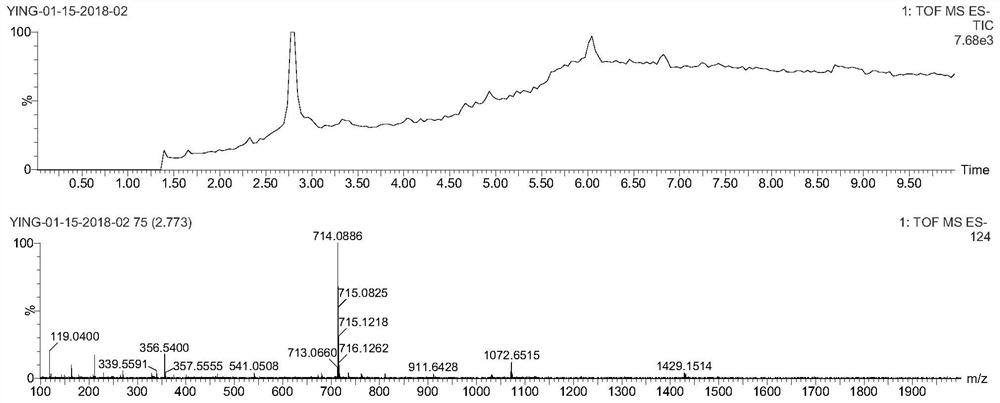
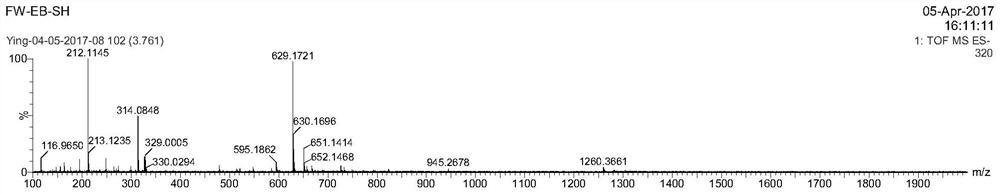
[0078] In a 20mL reaction bottle, add 10mg (1eq) of EB-NH prepared according to the method of Example 1 2 , 3,3'-thiodipropionic acid (7.8mg, 10eq), benzotriazol-1-oxytri(pyrrolidino)phosphonium hexafluorophosphate (PyBOP, 19mg, 2eq), DIPEA (23.8, 10eq) In 5mL DMF, reacted for 24 hours, then purified by preparative HPLC using acetonitrile and 0.1% trifluoroacetic acid aqueous solution (gradient: 5-95% acetonitrile), the purified solution was directly added excess tris(2-carboxyacetate) phosphine hydrochloride (TECP), reacted for 4 hours, then purified by preparative HPLC using acetonitrile and 0.1% aqueous trifluoroacetic acid (gradient: 5-95% acetonitrile), and freeze-dried to obtain EB1-SH. LC-MS confirmed the structure as image 3 , ESI-MS m / z: calculated 630.09, found 629.17 (M-H).
[0079] In a 20 mL reacti...
Embodiment 3
[0080] Embodiment 3: Preparation of EB-DM1
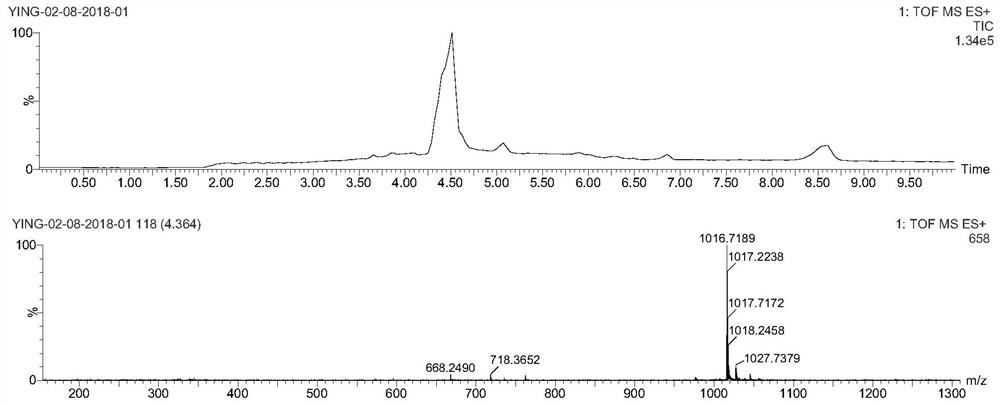
[0081] In a 20 mL reaction vial, DM1 (5.3 mg, 7.2 μmol) was weighed and dissolved in 2 mL DMF, then EB-Mal (2.5 mg, 3.6 μmol, 2 mL PBS) was added. The reaction solution was shielded from light with aluminum foil and stirred overnight at room temperature, and then purified by preparative HPLC using acetonitrile and 0.1% aqueous trifluoroacetic acid (gradient: 5-95% acetonitrile) to obtain EB-DM1. The collected purified product was lyophilized and stored at -20°C for later use. Yield: 2.7 mg (52% yield). ESI-MS m / z: calculated value 1431.40, measured value 1430.57 (M-H) ( Figure 5 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com