Application of licochalcone A in preparation of anti-anaphylactoid drugs
A technology for licorice chalcone and allergic reactions, applied in the field of biomedicine, to reduce local and systemic allergy-like symptoms, improve symptoms, and reduce mast cell degranulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
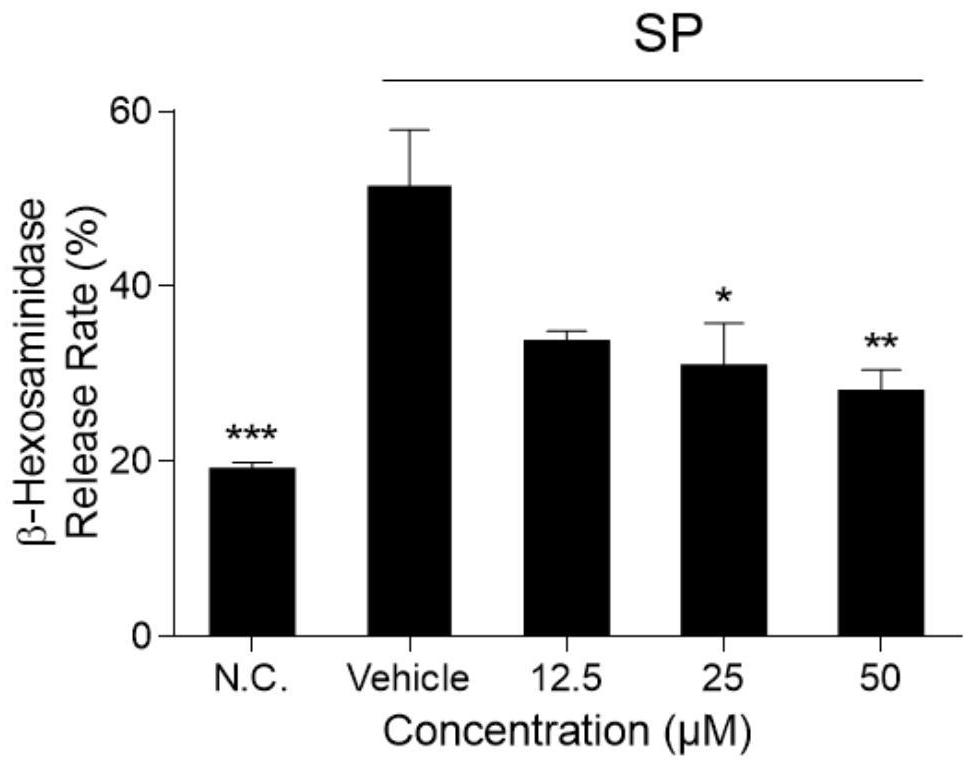
[0028] Example 1 Licochalcone A inhibits SP-induced KU812 cell β-hexosaminidase release
[0029] 1. Experimental materials
[0030] SP (purchased from MCE Company), KU812 cells, RPMI1640 medium (containing penicillin 100 U / mL, streptomycin 0.1 mg / mL, 20% FBS), Tyrode's solution, licochalcone A (purchased from Baoji Chenguang Company) , 0.1MNa 2 CO 3 , 0.1M NaHCO 3 , β-hexosaminidase, 0.1M citric acid, 0.1M sodium citrate.
[0031] The SP (Substance P) used refers to the neuropeptide discovered by Von Euler and Gaddum in 1931, the sequence is Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH 2 , the molecular weight is 1340.
[0032] Further, the purity of SP is ≥97%.
[0033] The structural formula of the licochalcone A is as follows:
[0034]
[0035] 2. Experimental method
[0036] KU812 cells were seeded in 96-well plates (1×10 5 per well), and the culture medium was discarded by centrifugation. Licochalcone A solutions (concentrations of 0 μM, 12.5 μM, 25 μM and ...
Embodiment 2
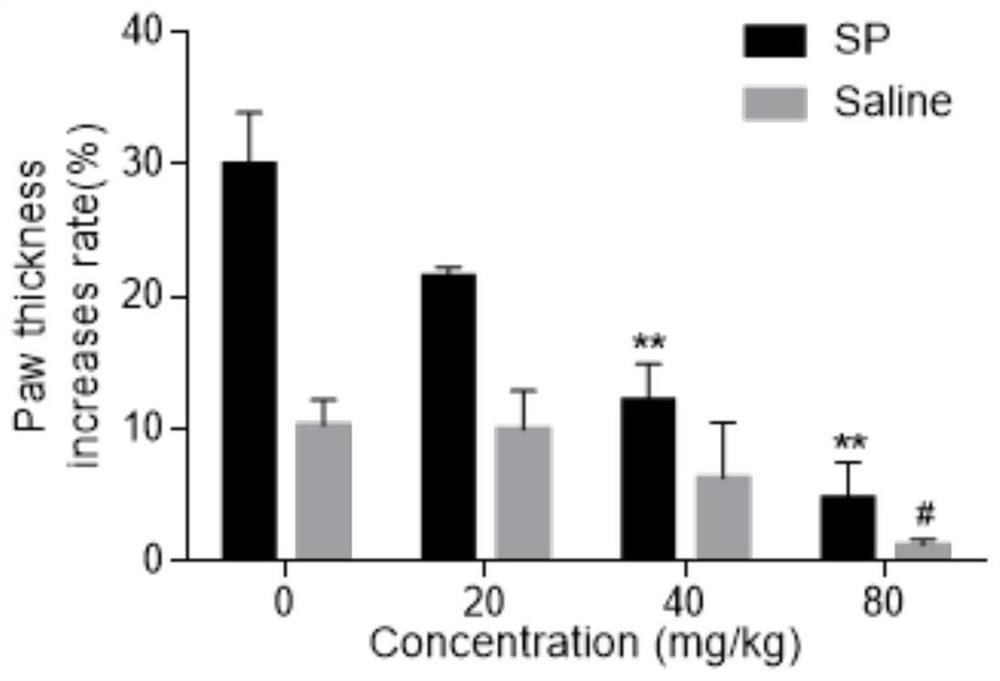
[0040] Example 2 Licochalcone A inhibits mouse toe swelling and Evans blue exudation caused by SP
[0041] 1. Experimental materials
[0042] SP (purchased from MCE Company), 6-8 week-old male C57 mice (purchased from the Experimental Animal Center of Xi'an Jiaotong University), licochalcone A (purchased from Baoji Chenguang), 0.4% Evans blue solution, 3.5% hydrated Chloral solution, PBS solution, normal saline, acetone.
[0043] 2. Experimental method
[0044]The mice were divided into blank control group, positive control group and licochalcone A administration groups of various concentrations. Licochalcone A solutions of different concentrations (respectively 20 mg / kg, 40 mg / kg, 80 mg / kg) were prepared with 0.5% CMC-Na solution for intragastric administration. After 30 minutes, mice were anesthetized with 3.5% chloral hydrate solution, and 0.4% Evans blue solution was injected into the tail vein. The thickness of the left and right soles of the mice in each group was me...
Embodiment 3
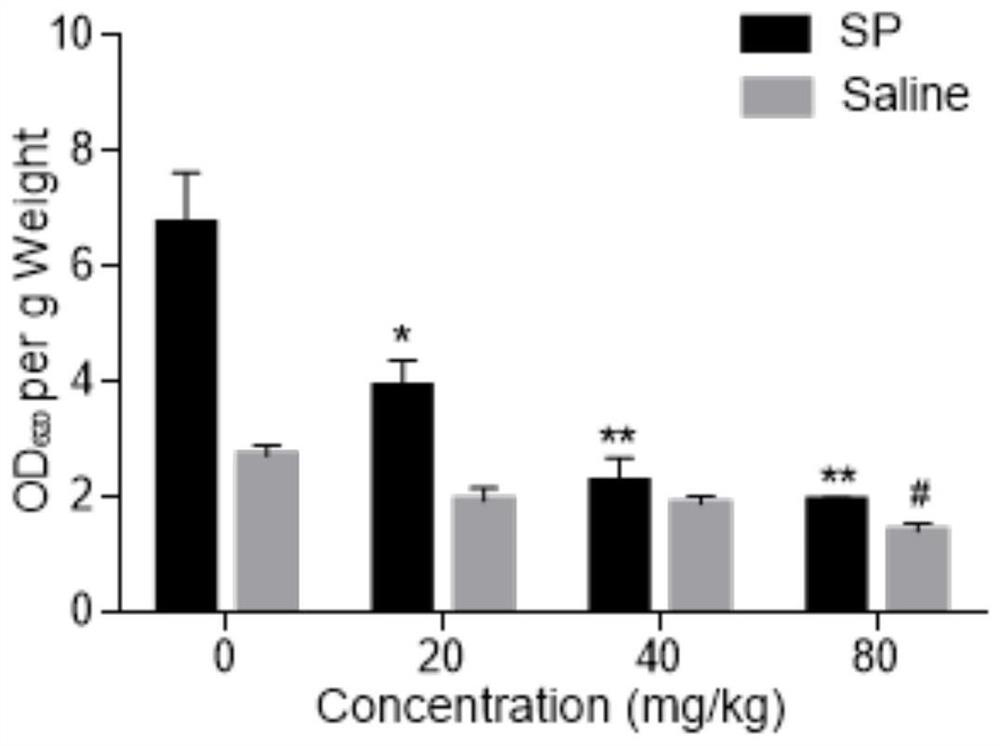
[0047] Example 3 Licochalcone A inhibits the increase of serum TNF-α content in mice caused by SP
[0048] 1. Experimental materials
[0049] SP (purchased from MCE Company), 6-8 week-old male C57 mice (purchased from the Experimental Animal Center of Xi'an Jiaotong University), licochalcone A (purchased from Baoji Chenguang), mouse TNF-αELISA kit (purchased from Beijing Yiqiao).
[0050] 2. Experimental method
[0051] The mice were divided into blank control group, positive control group and licochalcone A administration groups of various concentrations. Licochalcone A solutions of different concentrations (respectively 20 mg / kg, 40 mg / kg, 80 mg / kg) were prepared with 0.5% CMC-Na solution for intragastric administration. After 30 minutes, 30 μg / mL SP solution was injected into the tail vein, and the blank control group was injected with an equal volume of normal saline. After 1 hour, blood was collected, centrifuged at 10,000 g for 10 minutes, and serum was collected.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com