Application of Licochalcone A in preparation of antiallergic drug
A licorice chalcone and anti-allergic technology, which is applied in drug combinations, allergic diseases, pharmaceutical formulations, etc., can solve problems such as the application of anti-allergic drugs that have not been reported, and achieve the reduction of mast cell degranulation, improvement of symptoms, and relief of local and systemic allergy reaction symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
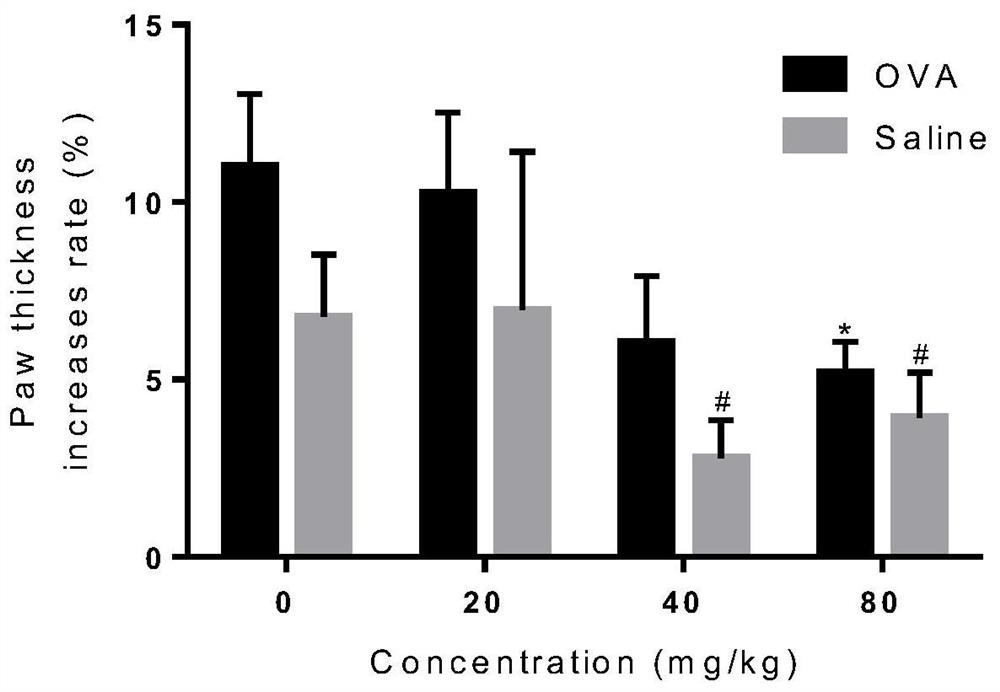
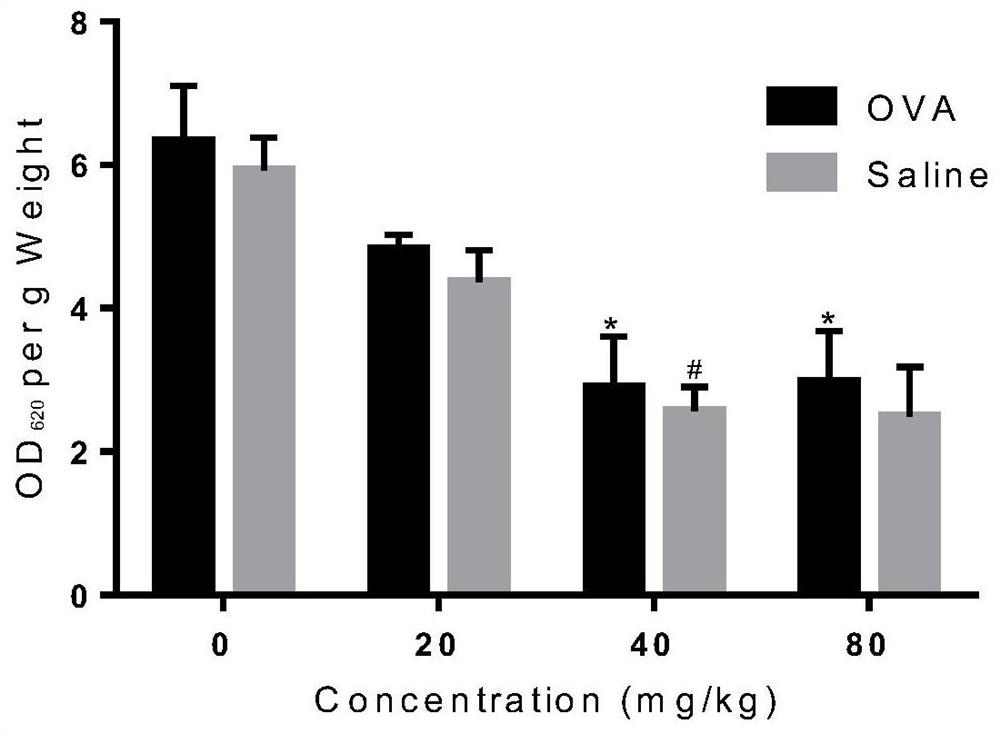
[0025] Example 1 Licochalcone A inhibits mouse toe swelling and Evans blue exudation caused by OVA
[0026] 1. Experimental materials
[0027] Ovalbumin (OVA was purchased from SIGMA Company), 6-8 week-old male C57 mice (purchased from the Experimental Animal Center of Xi'an Jiaotong University), licochalcone A (purchased from Baoji Chenguang), 0.4% Evans blue solution, 3.5% chloral hydrate solution, PBS solution, normal saline, acetone.
[0028] The structural formula of the licochalcone A is as follows:
[0029]
[0030] 2. Experimental method
[0031] The mice were divided into blank control group, positive control group and licochalcone A administration groups of various concentrations. The rats were sensitized by intraperitoneal injection of 20 μg / mL OVA solution one week in advance. Licochalcone A solutions of different concentrations (respectively 20 mg / kg, 40 mg / kg, 80 mg / kg) were prepared with 0.5% CMC-Na solution for intragastric administration. After 30 minu...
Embodiment 2
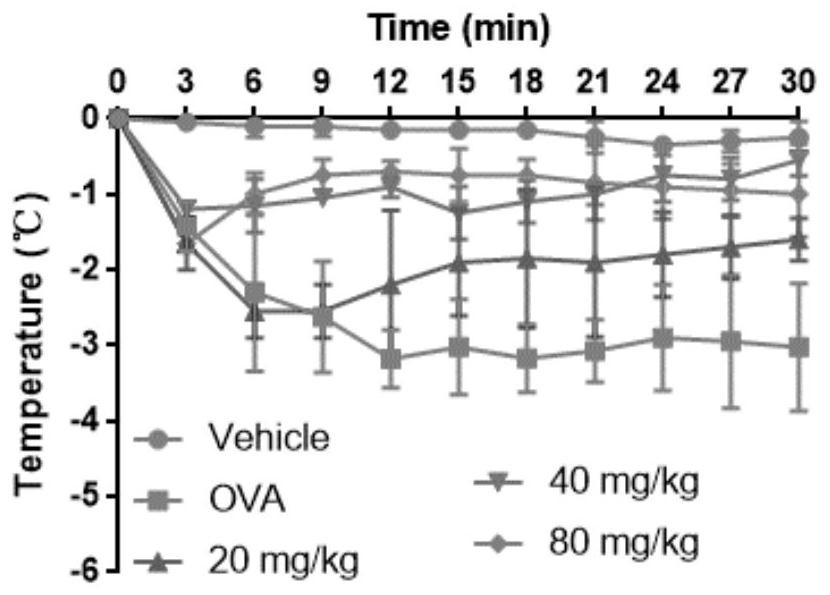
[0034] Example 2 Licochalcone A inhibits OVA-induced hypothermia in mice
[0035] 1. Experimental materials
[0036] OVA (purchased from SIGMA Company), 6-8 week-old male C57 mice (purchased from the Experimental Animal Center of Xi'an Jiaotong University), licochalcone A (purchased from Baoji Chenguang), and a mouse thermometer.
[0037] 2. Experimental method
[0038] The mice were divided into blank control group, positive control group and licochalcone A administration groups of various concentrations. The rats were sensitized by intraperitoneal injection of 20 μg / mL OVA solution one week in advance. Licochalcone A solutions of different concentrations (respectively 20 mg / kg, 40 mg / kg, 80 mg / kg) were prepared with 0.5% CMC-Na solution for intragastric administration. After 30 minutes, 100 μg / mL OVA solution was injected into the tail vein, and the blank control group was injected with an equal volume of normal saline. The rectal temperature of the mouse was detected ev...
Embodiment 3
[0041] Example 3 Licochalcone A inhibits mouse mast cell degranulation
[0042] 1. Experimental materials
[0043] Male C57 mice aged 6-8 weeks (purchased from the Experimental Animal Center of Xi'an Jiaotong University), Licochalcone A (purchased from Baoji Chenguang), and mouse β-aminoglycoside substrate kit (purchased from Beijing Yiqiao).
[0044] A fully automatic microplate reader was purchased from Bio-Rad (California, USA).
[0045] Cell line: Mouse peritoneal mast cell PBMC cultured in 5% CO 2 , in an environment of 37°C. The medium was half-replaced with fresh medium every week to maintain the cells at 2 × 10 6 density of cells per milliliter. TM buffer solution (composition (g / L): 6.954 NaCl, 0.353 KCl, 2.383 HEPES, 0.162 KH 2 PO 4 , 0.282 CaCl 2, 0.143 MgSO 4 , 0.991 glucose, 1 bovine serum albumin. Triton X-100 was purchased from Kehao Bioengineering Company (Xi'an, China), D-glucose Tianjin Kemiou Chemical Reagent Company (Tianjin, China), bovine serum a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com