Therapeutic drug targeting fibroblast activating protein and preparation method thereof
A therapeutic drug and a fibroblast-oriented technology, which is applied in the field of therapeutic drugs targeting fibroblast activation protein and its preparation and labeling, and can solve the problems of unsatisfactory therapeutic use, inability to achieve mass production and distribution, and short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Preparation of compound DOTA-tEB-FAPI02
[0033] Synthesis of compound 4:
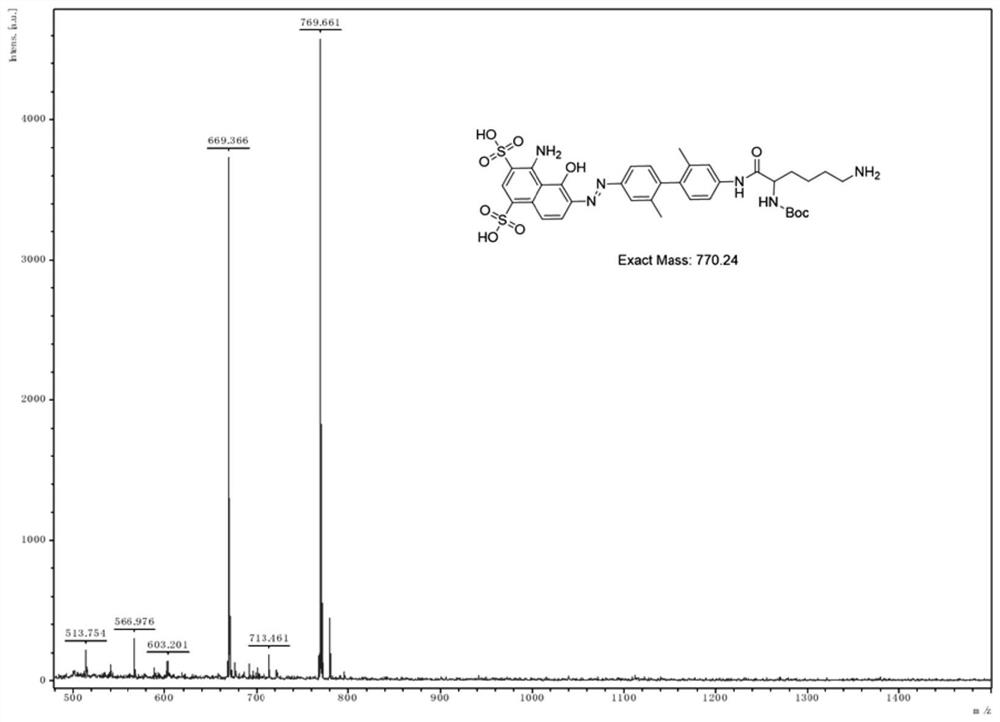
[0034] Add COOH-PEG to the N,N-dimethylformamide of compound 3 (1.0mmol) 2 -COOH (1.50mmol), HATU (1.0mmol) and N,N-diisopropylethylamine (3.0mmol), stirred at room temperature overnight, and the completion of the reaction was monitored by HPLC. The solvent was distilled off under reduced pressure to obtain a crude product. The crude product was reverse-phase columnized and freeze-dried to obtain pure compound 4 with a yield of 67%. Among them, the mass spectrum of compound 3 is shown in figure 1 .
[0035] Synthesis of compound 5:
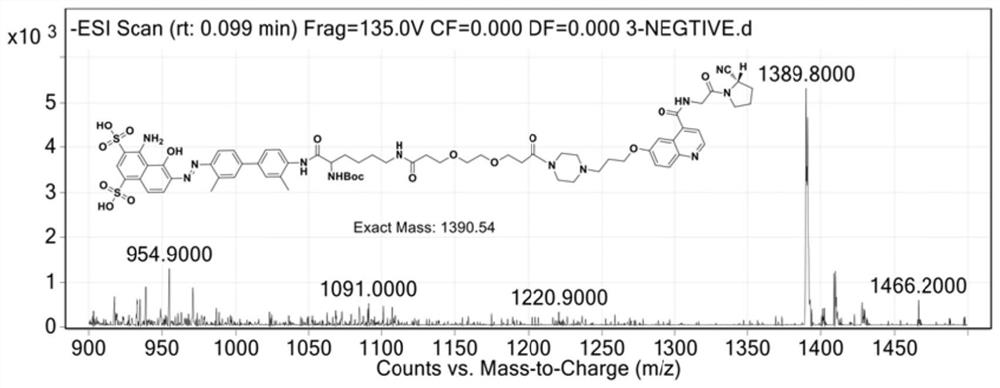
[0036] Put compound 4 (0.1mmol), HATU (0.1mmol) and N,N-diisopropylethylamine (0.3mmol), FAPI small molecule inhibitor compound 1 (0.1mmol) and 5mL N,N into a 50mL flask respectively. -Dimethylformamide, react at room temperature, and monitor the completion of the reaction by HPLC. The solvent was distilled off under reduced pressure to obtain a cr...
Embodiment 2
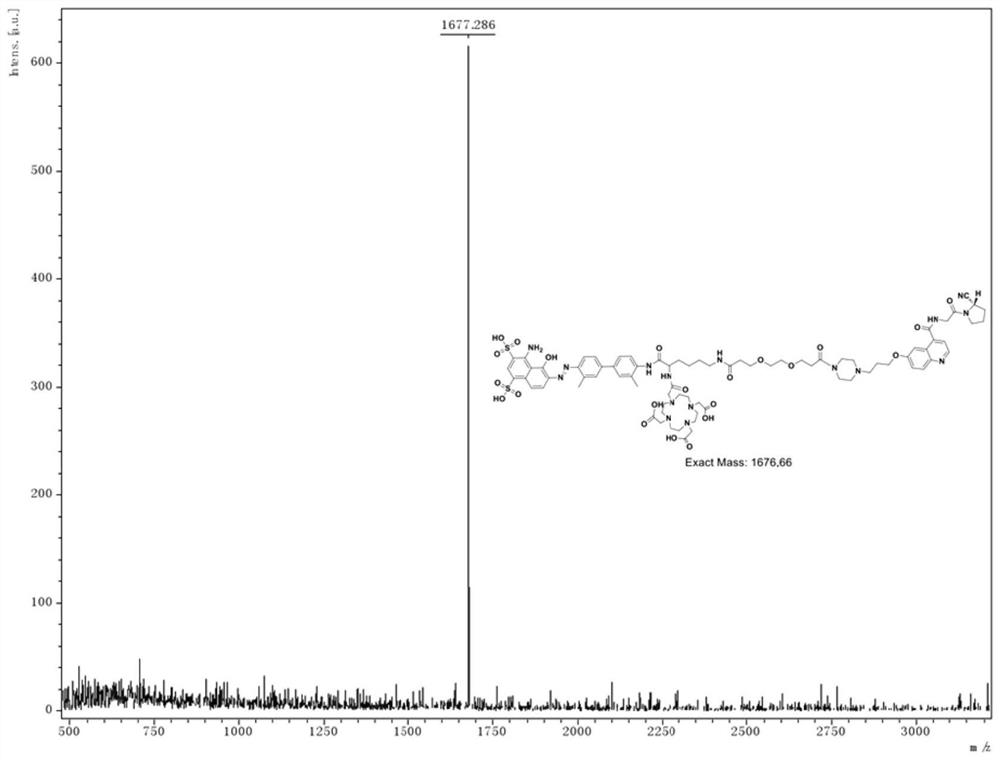
[0042] Example 2. Preparation of 177Lu-DOTA-tEB-FAPI02 complex:
[0043] Dissolve 50 micrograms of the compound DOTA-tEB-FAPI02 prepared by the method in Example 1 in 20 microliters of DMSO, add 200 microliters of buffer solution (pH=5.5), oscillate to dissolve it completely, and add about 5 mCi 177 LuCl 3. The mixture was shaken and heated at 95°C for 30 min. Cool to room temperature after the reaction. Take a C18 separation column, wash it slowly with 10mL of absolute ethanol, and then with 10mL of water. After diluting the labeling solution with 10mL water, load the sample on the separation column, first remove the unlabeled solution with 10mL water 177 Lu ions, and then rinsed with 0.3mL 10mM HCl ethanol solution to obtain the product. The eluate was diluted with normal saline and sterile filtered to obtain 177 Injection of Lu-DOTA-tEB-FAPI02. HPLC quality control results such as Figure 4 shown.
experiment example
[0044] Experimental example. Analysis and application effect
[0045] 1. HPLC analysis and identification
[0046] The HPLC system was as follows: SHIMADZULC-20A; C18 chromatographic column (YMC, 3 μm, 4.6×150 mm) was used for analysis. Detection wavelength 254nm, flow rate 1mL / min, eluting gradient: 0-3 minutes: 10% acetonitrile (0.1% TFA) and 90% water (0.1% TFA) remain unchanged; 3-16 minutes: increase to 90% acetonitrile (0.1% TFA) and 10% water (0.1% TFA); 16-18min: reduce to 10% (0.1% TFA) and 90% water (0.1% TFA); 18-20min: maintain 10% acetonitrile (0.1% TFA ) and 90% water (0.1% TFA).
[0047] 2, 177 Experiments of Lu-DOTA-tEB-FAPI02 complex in normal mice and U87 tumor-bearing model mice.
[0048] Prepared by the method of Example 2 with a purity greater than 95% 177 Lu-DOTA-tEB-FAPI02, in normal mice and U87 tumor-bearing model mice, injected 1.3MBq via tail vein 177 Lu-tEB-FAPI. Pharmacokinetics and SPECT imaging were tested at different time points after in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com