Method for preparing rare ginsenoside by hydrolyzing ginsenoside with acidic amino acid
A technology of ginseng rare saponins and acidic amino acids, which is applied in the fields of organic chemistry and steroids, can solve the problems of strong corrosion, poor hydrolysis specificity and low yield of ginsenosides, and achieve low-cost enrichment and overcome hydrolysis specificity. one-off effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
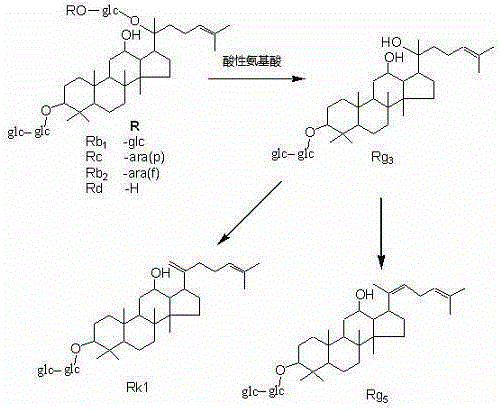
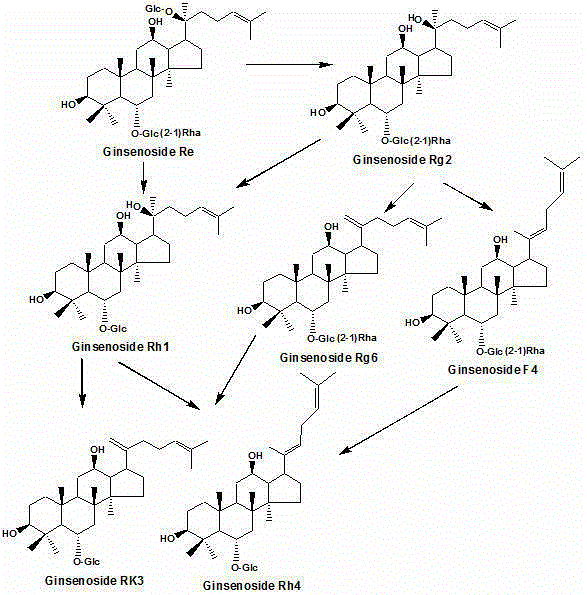
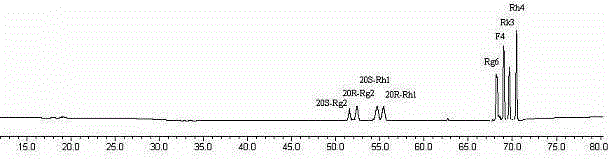
[0020] Panaxadiol group saponins or triol group saponins are dissolved in water according to the mass volume ratio of 1:1 (kg / liter), and acidic amino acids are added, so that the mass ratio of panaxadiol group saponins or triol group saponins to acidic amino acids is 1:10 (kg / kg); then put it in a pressure cooker and cook at 80°C for 1 hour, take out the cooked hydrolyzate and put it on the macroporous resin column D 101, first washed with water for five column volumes, and then eluted with 60% ethanol. After recovering ethanol, the hydrolyzed product of ginseng diol group saponins obtained rare ginsenoside 20R-Rg 3 、20S-Rg 3 , Rg 5 and Rk1( figure 1 and image 3 ); Panaxatriol group saponin hydrolyzate obtains rare ginsenoside Rg 6 , F4, Rk3 and Rh4 ( figure 2 and Figure 4 ).
Embodiment 2
[0022] Panaxadiol group saponins or triol group saponins are dissolved in water according to the mass volume ratio of 1:10 (kg / liter), and acidic amino acids are added, so that the mass ratio of panaxadiol group saponins or triol group saponins to acidic amino acids is 1:1 (kg / kg); then put it in a pressure cooker and cook at 100°C for 2 hours, take out the cooked hydrolyzate and put it on the macroporous resin column D 101 , first washed with water for five column volumes, and then eluted with 70% ethanol. After recovering ethanol, the hydrolyzed products of ginsenosides of ginseng diol group obtained rare ginsenoside 20R-Rg 3 、20S-Rg 3 , Rg 5 and Rk1( figure 1 and image 3 ); Panaxatriol group saponin hydrolyzate obtains rare ginsenoside Rg 6 , F4, Rk3 and Rh4 ( figure 2 and Figure 4 ).
Embodiment 3
[0024] Panaxadiol group saponins or triol group saponins are dissolved in water according to the mass volume ratio of 1:20 (kg / liter), and acidic amino acids are added, so that the mass ratio of panaxadiol group saponins or triol group saponins to acidic amino acids is 20:1 (kg / kg); then put it in a pressure cooker and cook at 120°C for 4 hours, take out the cooked hydrolyzate and put it on the macroporous resin column D 101 , first washed with water for five column volumes, and then eluted with 80% ethanol. After recovering ethanol, the hydrolyzed products of ginsenosides of ginseng diol group obtained rare ginsenoside 20R-Rg 3 、20S-Rg 3 , Rg 5 and Rk1( figure 1 and image 3 ); Panaxatriol group saponin hydrolyzate obtains rare ginsenoside Rg 6 , F4, Rk3 and Rh4 ( figure 2 and Figure 4 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com