Method for reforming terpene-based synthase
A technology of terpene synthase and nucleic acid molecules, which is applied in the biological field and can solve problems such as the development of terpene synthase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
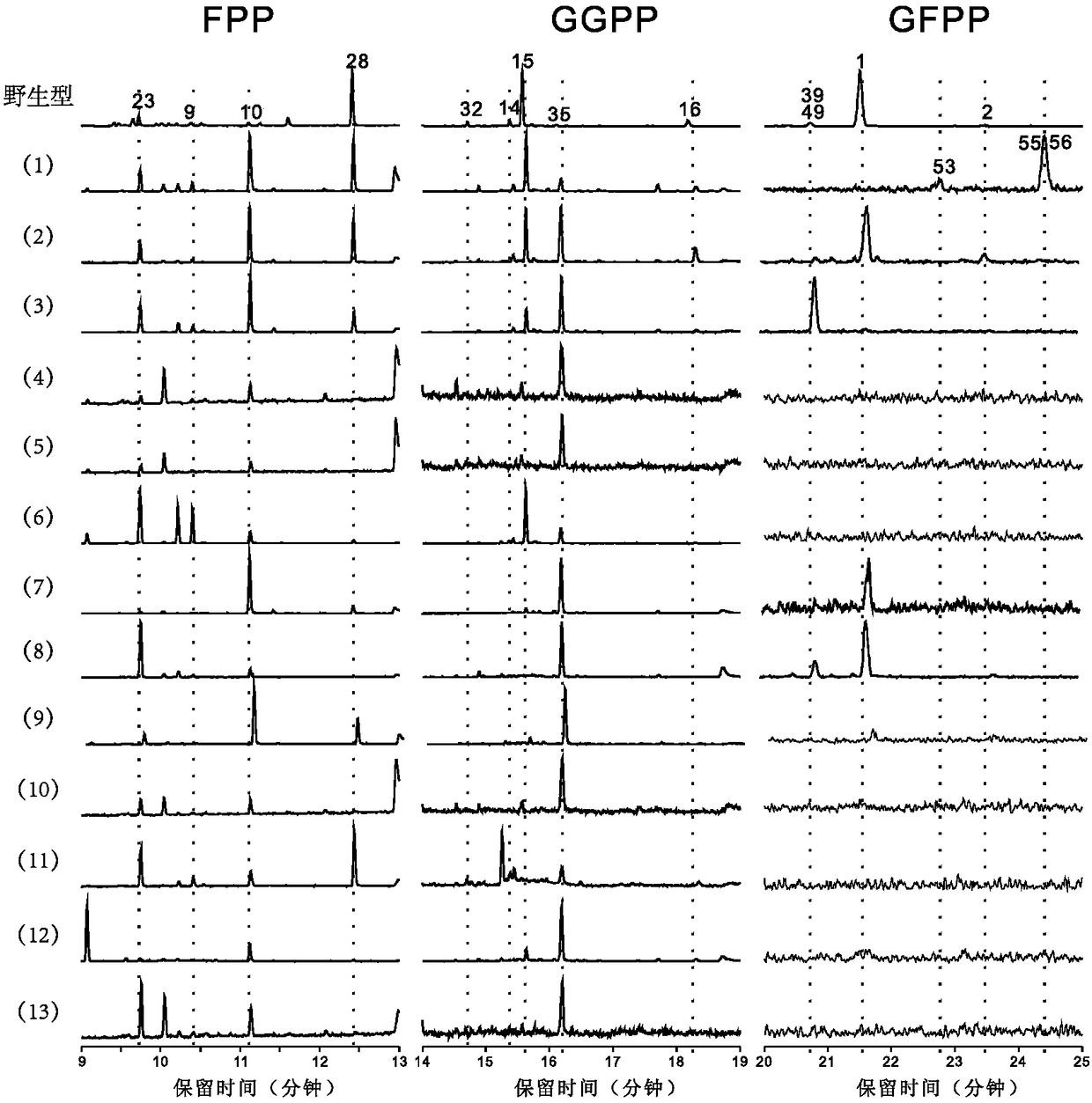
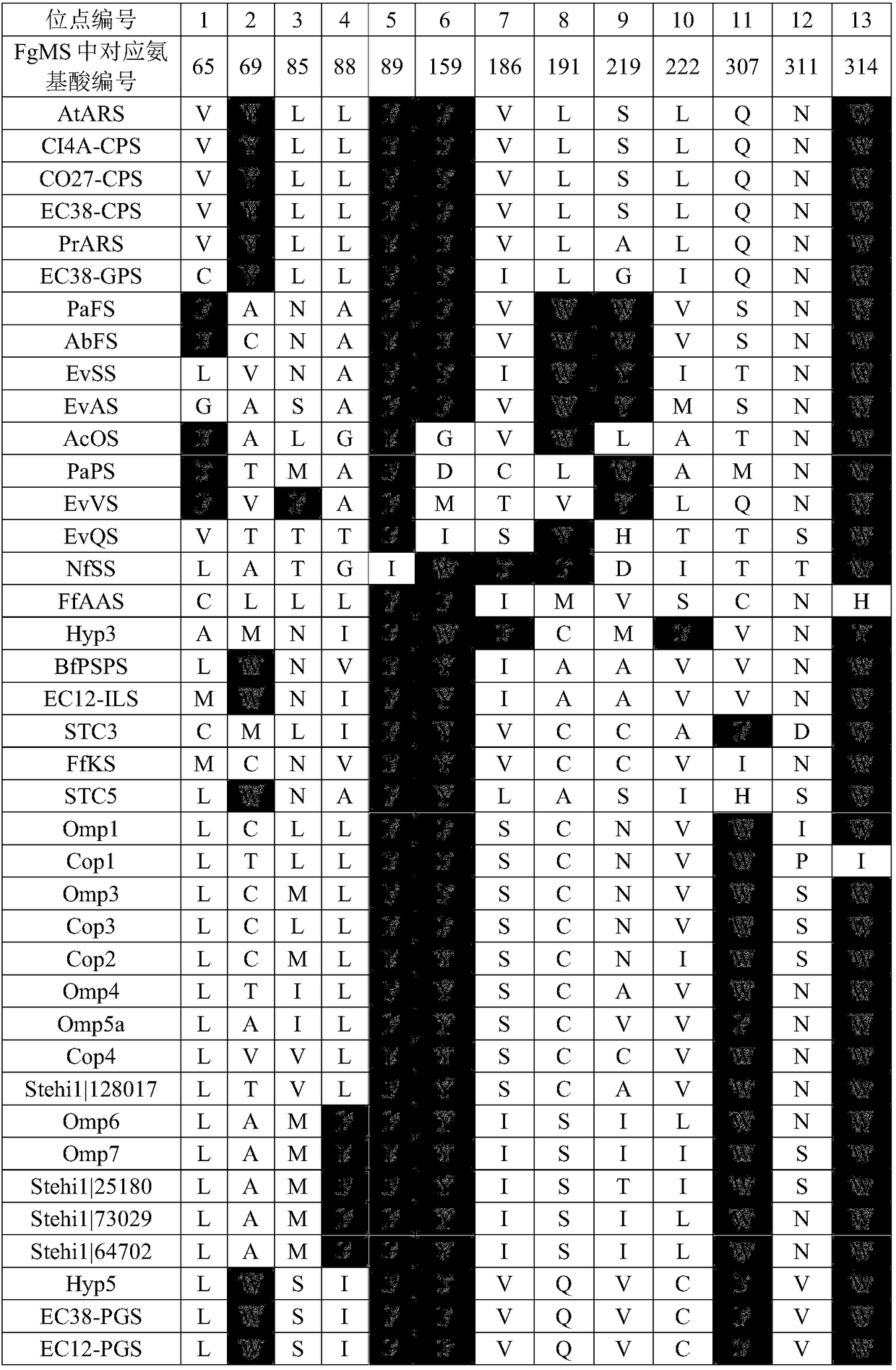
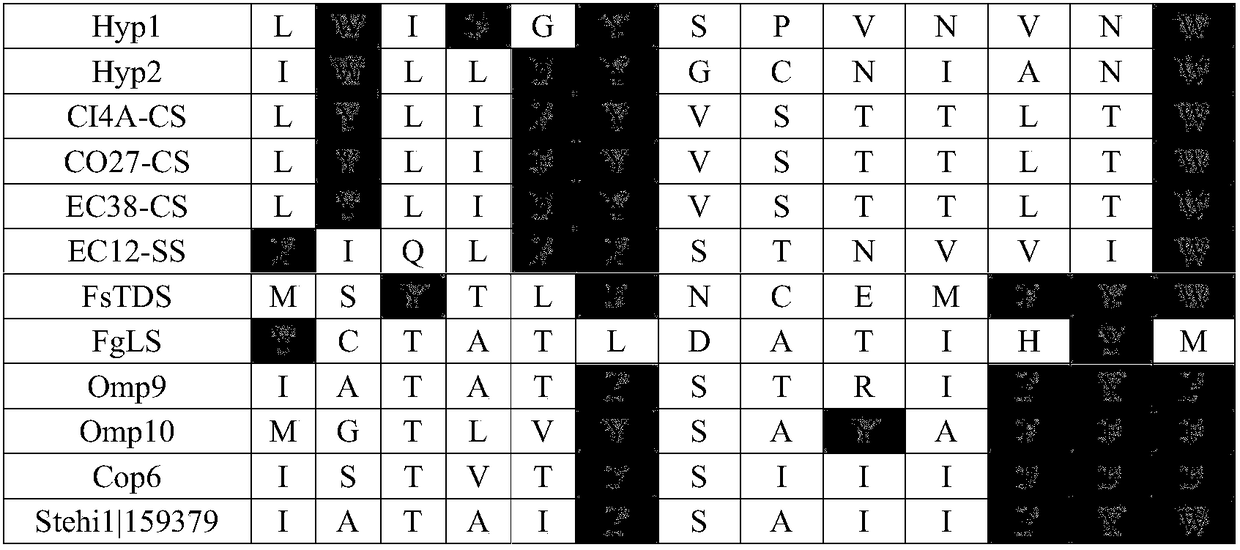
[0069] A multiple sequence alignment was performed on the sequences of 51 fungal terpene synthases whose functions were clearly verified in the literature reports before August 2016. Subsequently, the substrate-binding regions of these proteins were analyzed in combination with the representative crystal structure data, and the highly conserved catalytic-related sites were excluded to obtain the amino acid sites that determine functional specificity (Table 1). These correspond to the 65th, 69th, 85th, 88th, 89th, 159th, 186th, 191st, 219th, 222nd, 307th, 311th, 314th positions in the terpene synthase FgMS, There are 13 positions in total. The gray part is the aromatic amino acid.
[0070] Table 1 Amino acid positions of terpene synthase
[0071]
[0072]
Embodiment 2
[0073] Example 2 Transformation of phenylalanine at position 65 of wild-type terpene synthase FgMS into leucine
[0074] In this example, the wild-type FgMs terpene synthase was mutated according to the following method, so that compared with the wild-type terpene synthase, the 65th phenylalanine was transformed into leucine :
[0075] The mutant gene F65L was obtained by mutating the 193rd-195th nucleotides of the nucleotide sequence shown in SEQ ID NO: 6 from TTT to CTG. After the gene was obtained, it was cloned into the expression vector of pet28a, and transformed into the expression host E.coli BL21(DE3). After transformation, single clones were picked and placed in LB medium containing corresponding antibiotics, and cultured overnight at 37°C and 220rpm. Transfer to 1L of fresh LB medium containing corresponding antibiotics according to 1% inoculum amount, and cultivate to OD at 37°C and 220rpm 600 About 0.6-0.8, lower the temperature to 16°C, add IPTG with a final co...
Embodiment 3
[0080] Example 3 Transformation of phenylalanine at position 89 of wild-type terpene synthase FgMS into leucine
[0081] According to the method of Example 2, the 265th-267th nucleotide of the nucleotide sequence shown in SEQ ID NO: 6 is mutated from TTT to CTG, so that the obtained terpene synthase is compared with the wild-type terpene synthase, The phenylalanine at position 89 was transformed into leucine.
[0082] Verification is carried out according to the mode of embodiment 2, and the results are shown in figure 1 (2), the following changes occurred in the product: 1. The content ratio of sesquiterpene main product 1 increased from 92.82% to 100%; 2. The content ratio of the original diterpene by-product 35 increased from 1.90% to 49.50%, becoming the main product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com