Self-assembly short peptides constructed by D type amino acid, use for nano-biomedicine
A technology of self-assembling short peptides and amino acids, which is applied in the field of nanobiomedicine, can solve the problems of limited application range and short nanofibers, and achieve the effects of improving the success rate, obvious social benefits and economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of self-assembled short peptide d-RAD16 composed of D-type amino acids
[0026] 1. Materials
[0027] Fmoc-D-Ala-OH (9-fluorenylmethoxycarbonyl-D-alanine), Fmoc-D-Asp(OtBu)-OH (fluorenylmethoxycarbonyl D-aspartic acid-ε-tert-butyl Oxycarbonyl), Fmoc-D-Asp(Boc)-Rink amide Resin (fluorenylmethoxycarbonyl-D-aspartic acid-ε-tert-butoxycarbonyl-Rink amide resin), Fmoc-D- Arg(pbf)-OH(9-fluorenylmethoxycarbonyl-D-arginine-γ-tert-butoxycarbonyl), HBTU(O-benzotriazol-1-yl-N,N,N,N -Tetramethylurine hexafluorophosphate) and HOBT (1-hydroxybenzotriazole) were purchased from Sichuan Shengxin Biological Pharmaceutical Co., Ltd.; piperidine, acetic anhydride, solvent: DMF (N, N-dimethyl Formamide), TFA (trifluoroacetic acid), DCM (dichloromethane), and NMM (N-methylmorpholine) were purchased from Chengdu Aofei Biochemical Co., Ltd.
[0028] 2. Preparation method
[0029] Adopt the solid-phase synthesis method of Fmoc (fluorenylmethoxycarbonyl) protection, pr...
Embodiment 2
[0047] Example 2: High-performance liquid chromatography and mass spectrometry detection and three-dimensional molecular model drawing of self-assembled short peptide d-RAD16
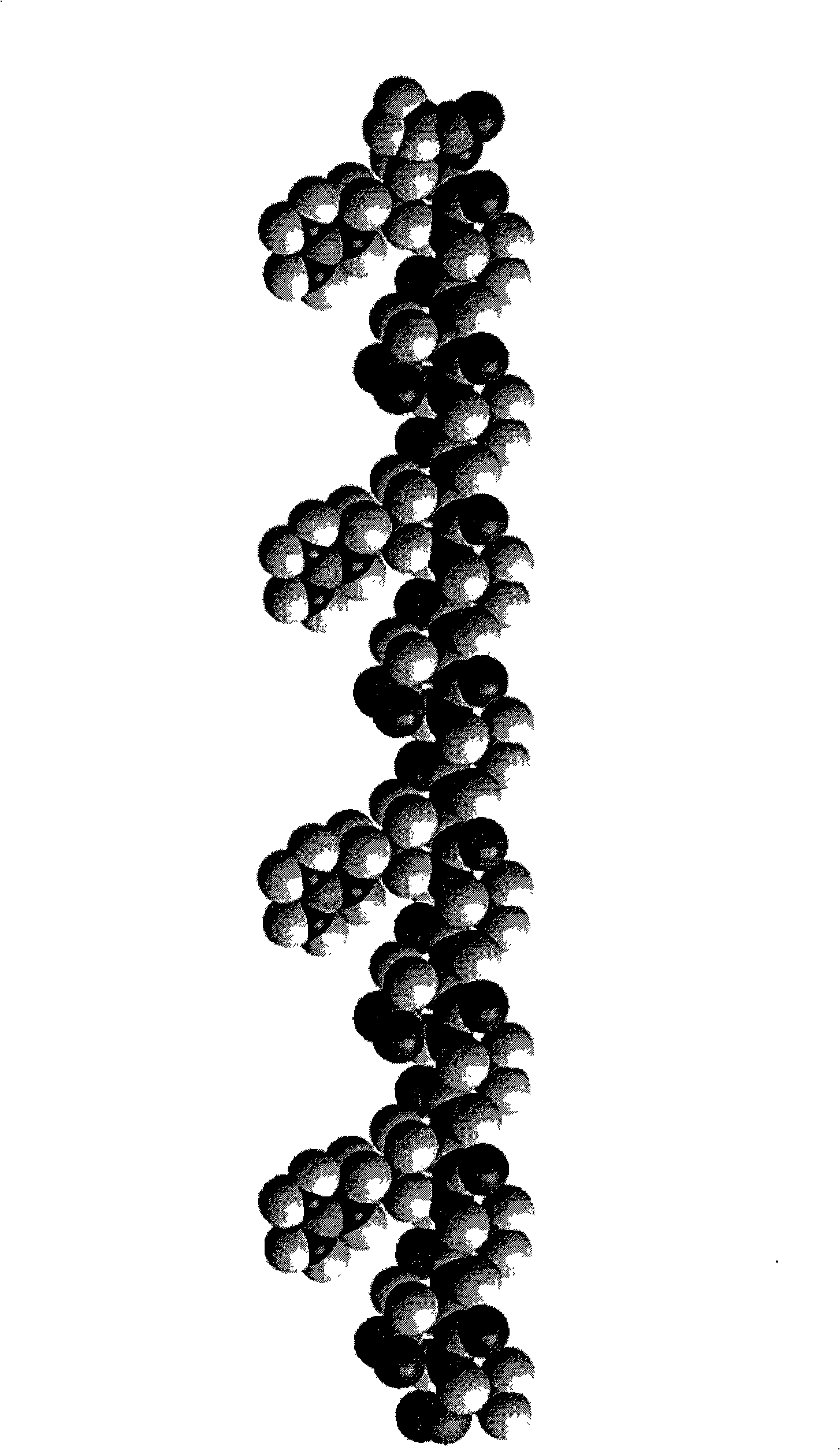
[0048] For the self-assembled short peptide d-RAD16 prepared in Example 1, use ordinary drawing software (Hyperchem7.5, www.hyper.com) to draw a schematic diagram of a three-dimensional molecular model based on the principle of energy minimization. The schematic diagram of the drawn three-dimensional molecular model is shown in figure 1 , through this schematic diagram, we can know the spatial distribution of its amino acids.
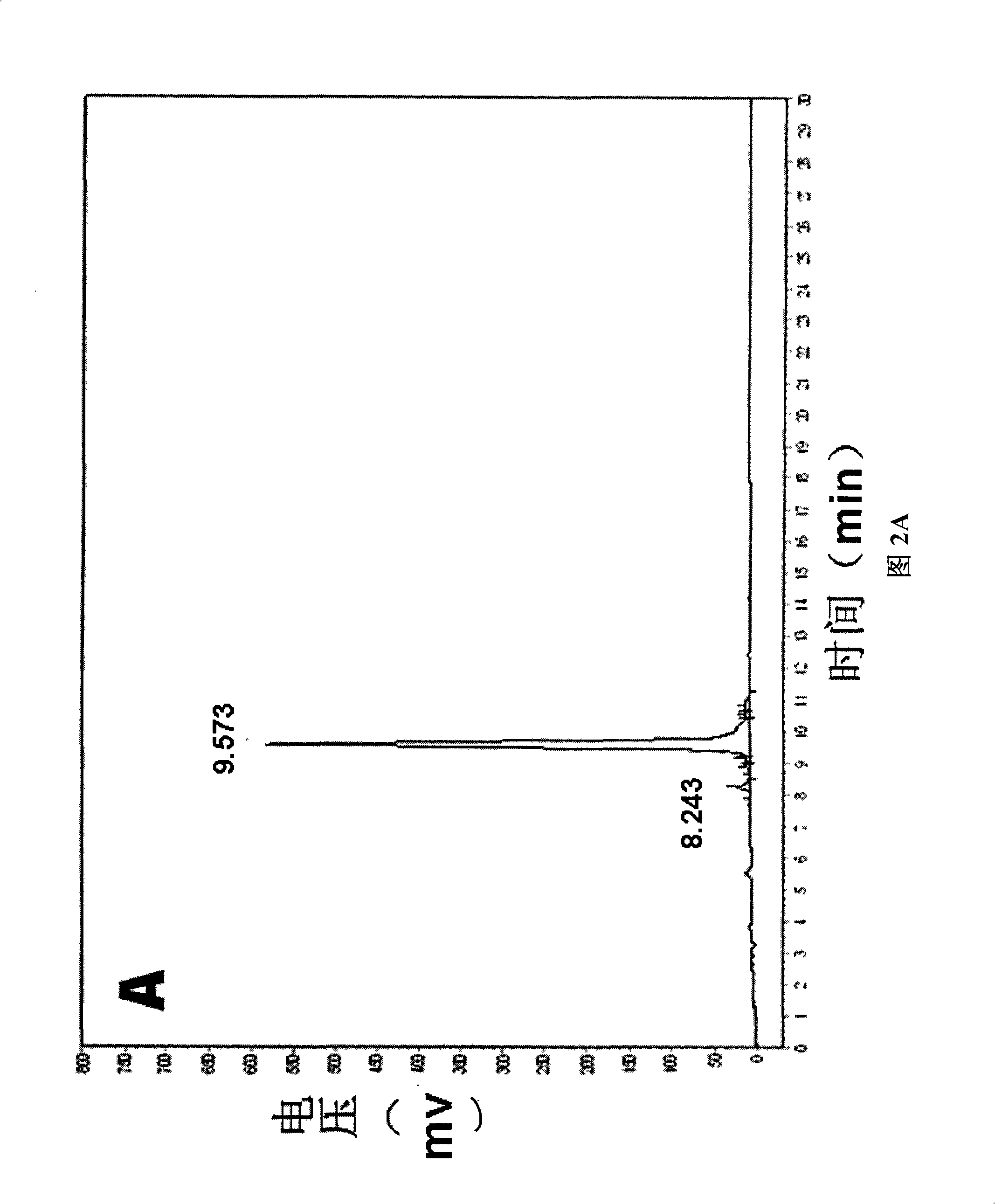
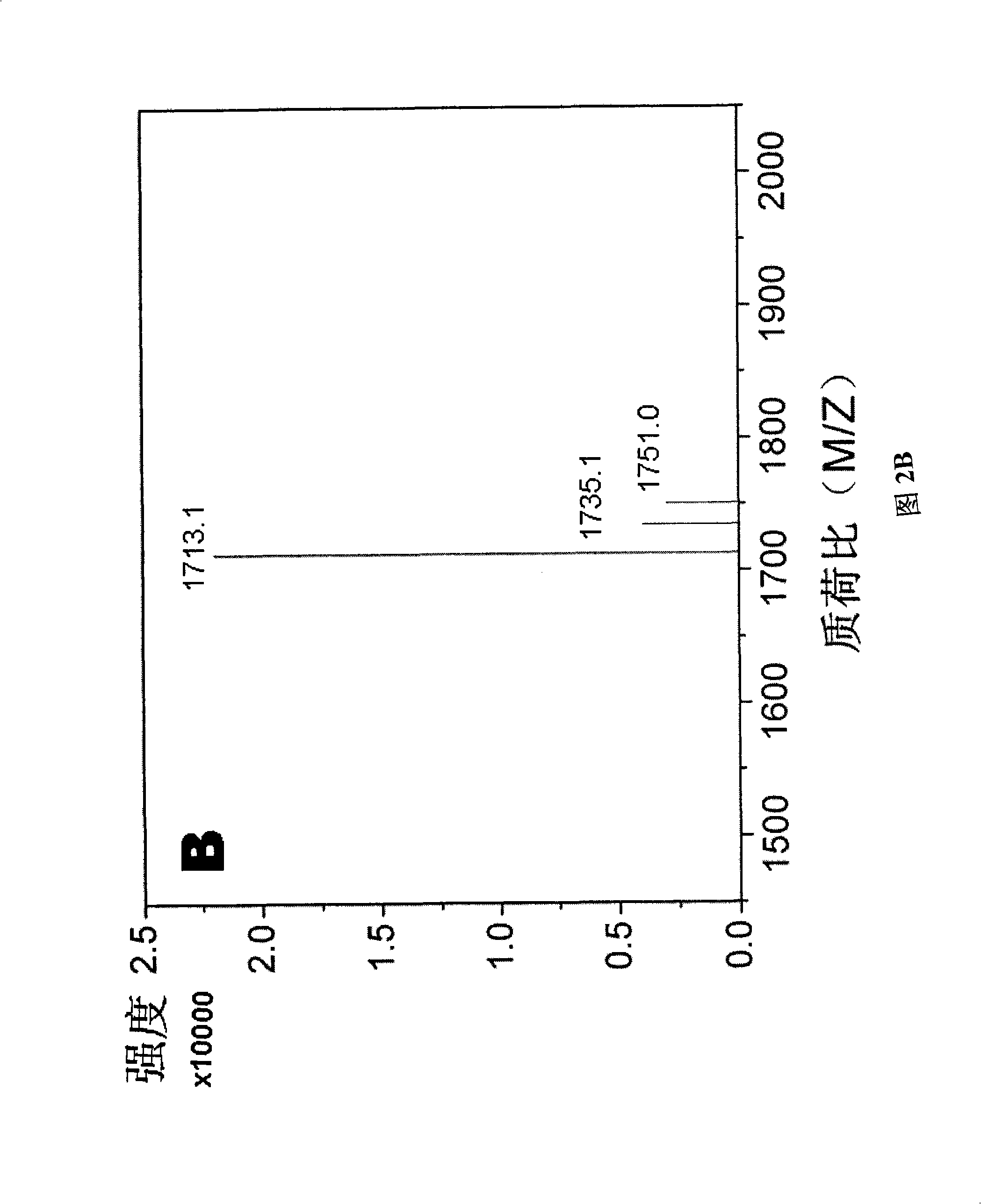
[0049] The self-assembled short peptide d-RAD16 prepared in Example 1 was detected by high-performance liquid chromatography (HPLC). The detection results are shown in FIG. 2A. According to the peak area in FIG. 2A, its purity reached 95%. The self-assembled short peptide d-RAD16 prepared in Example 1 was detected by mass spectrometry (MS). The detection results are shown in Figu...
Embodiment 3
[0050] Example 3: Self-assembled short peptide d-RAD16 forms hydrogel and water-holding performance
[0051] 1. Self-assembled short peptide d-RAD16 forms hydrogel under the action of different salt ions
[0052] (1) Take the short peptide d-RAD16 stored at 4°C to prepare a solution with a mass concentration of 1%, and use 18.2KΩ / cm 2 diluted with water to 500mmol / l or 1000mmol / l;
[0053] (2) Take Na + or K + Salt (such as NaCl, KCl) is prepared into salt solutions with different concentrations, and the concentration range of the salt solution is 0.001-1mol / l;
[0054] (3) Add an equal volume of salt solution to the short peptide d-RAD16 solution, and the self-assembly time is 24 hours.
[0055] 2. The self-assembled short peptide d-RAD16 forms a hydrogel under the physiological environment of cell growth
[0056] (1) Take 100 μl of culture medium (such as DMEM or MEM or RPMI-1640, etc.) containing 8-10% fetal bovine serum and drop it into a 96-well plate;
[0057] (2) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com