Preparation and method for treating diabetes
A diabetes and preparation technology, applied in the field of low-dose pharmaceutical preparations, can solve problems such as unsatisfactory blood sugar control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 and 2
[0123] Tablets containing the metformin / glibenclamide combination were prepared as described below.
[0124] Metformin hydrochloride-glibenclamide tablet composition
[0125] 250mg / 1.25mg and 500mg / 2.5
Embodiment 1 Embodiment 2
[0127]
[0128] * HPMC type film coating is used.
[0129] Metformin hydrochloride-glibenclamide tablet preparations 250mg / 1.25mg and 500mg / 2.5mg were compressed using the same granulation method. Compress small size tablets of half the weight of the 500mg / 2.5mg metformin hydrochloride-glibenclamide tablet. Tablets produced for clinical use are film coated with a hydroxypropylmethylcellulose (HPMC) film coating. The film coating is non-functional and suitable for anesthesia purposes. The abovementioned film coatings are known to be suitable for use in clinical preparations.
[0130] The production methods for processed clinical products are as follows:
[0131] The croscarmellose sodium and glibenclamide were dispersed together using a high shear mixer and then mixed with metformin hydrochloride / magnesium stearate (99.5%:0.5% w / w). The dry mixture obtained is granulated with an aqueous solution of polyvinylpyrrolidone in a high shear mixer and then dried in a fluid bed...
Embodiment 3
[0143] A. Overview of 5 clinical protocols
[0144] (1. Purpose
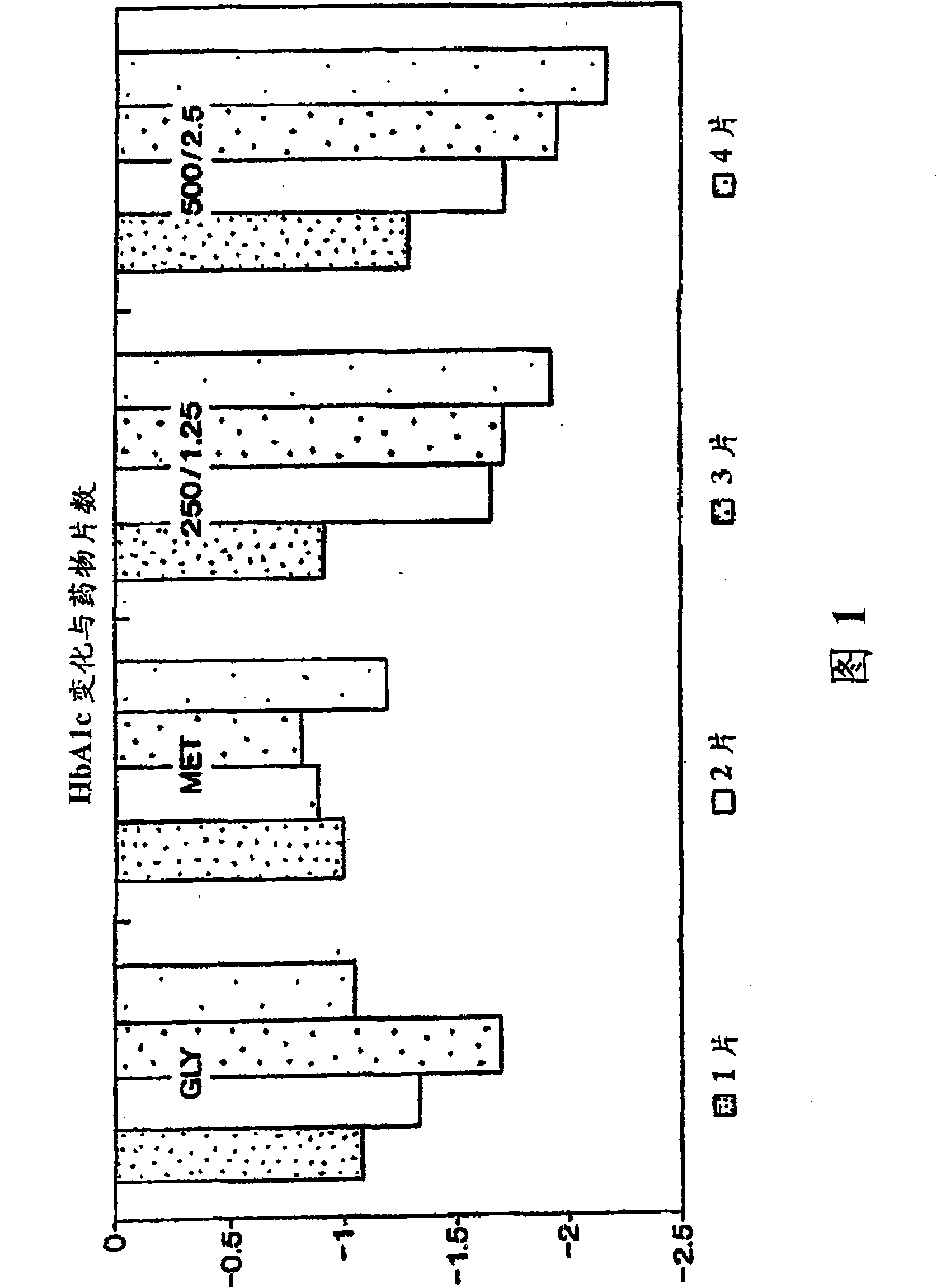
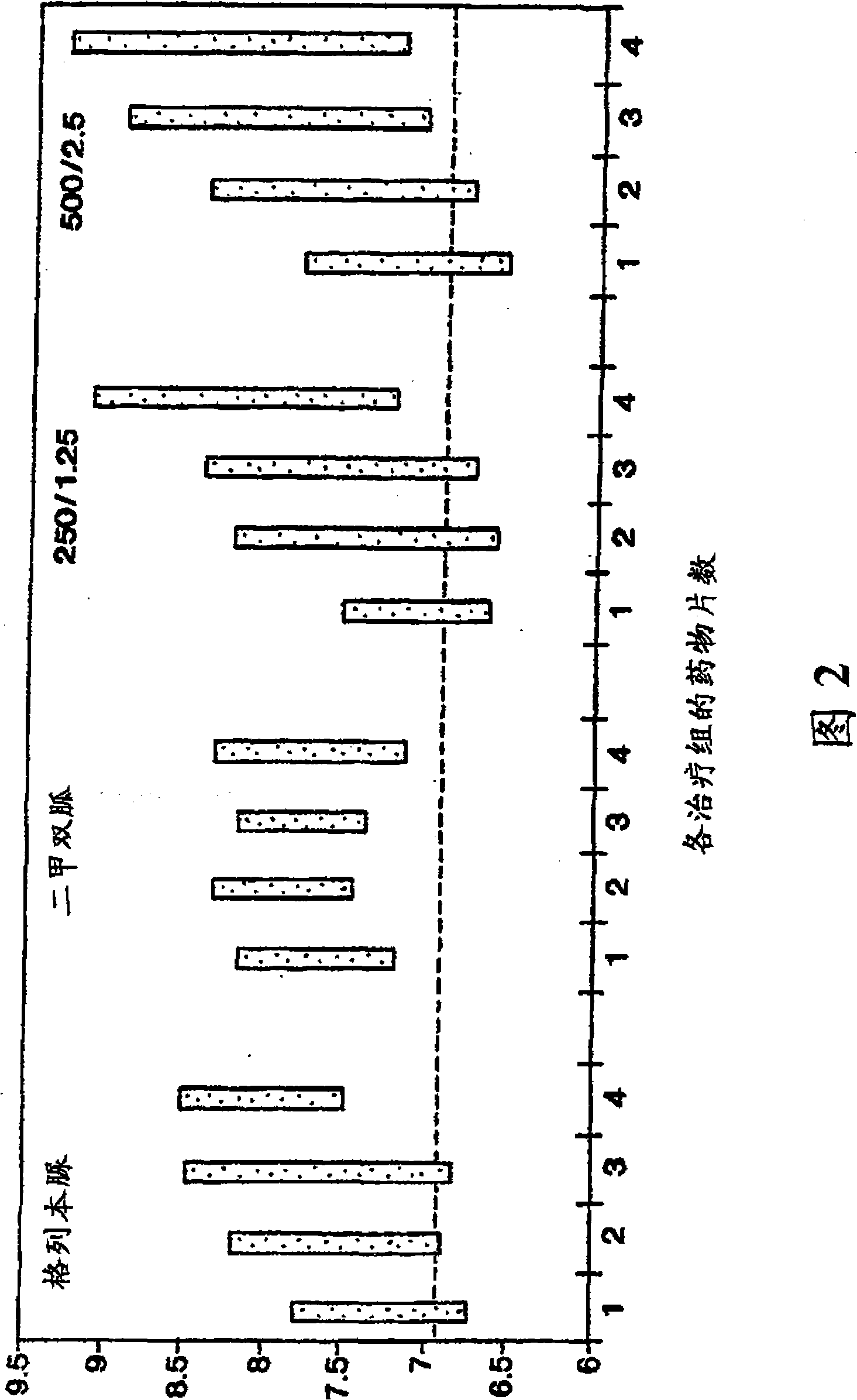
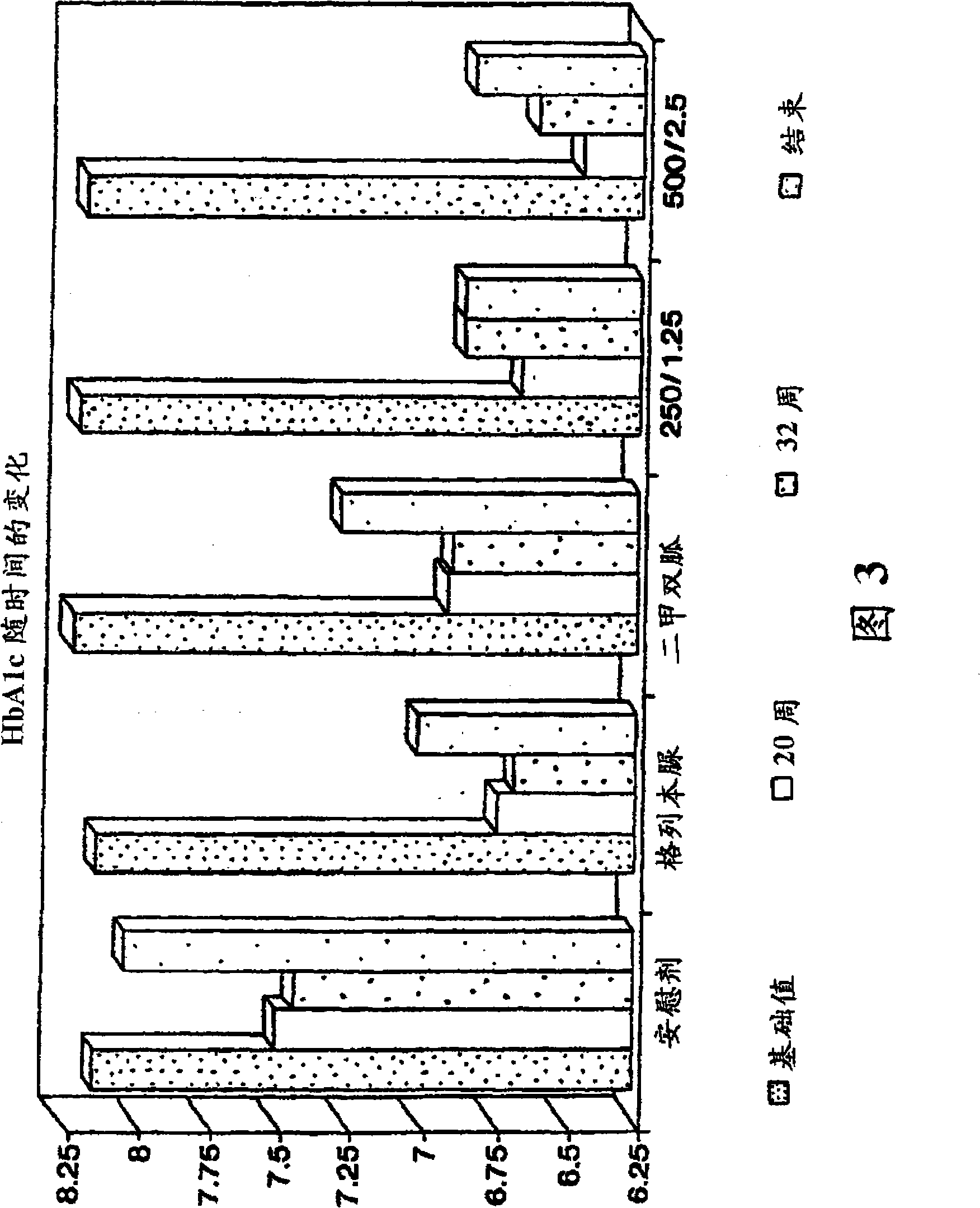
[0145] The purpose of the following study was to compare 2 dosage strengths of the fixed combination metformin / glibenclamide product (products described in Examples 1 and 2) with placebo in drug-naïve patients with type 2 diabetes who cannot control their blood sugar with diet and exercise. blood sugar control. Dosage strengths of the fixed combination drugs evaluated included 250 mg metformin and 1.25 mg glyburide, and 500 mg metformin and 2.5 mg glibenclamide. The most important standard measure of long-term blood glucose control is hemoglobin Alc (HbAlc) to evaluate blood glucose control. Compare the average change value of HbAlc after 20 weeks of treatment (4 weeks of stable daily medication, 4 weeks of gradually increasing dose and 12 weeks of stable administration). Treatment was continued for an additional 12 weeks in order to assess the durability of the effect.
[0146] The effects of the indivi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com