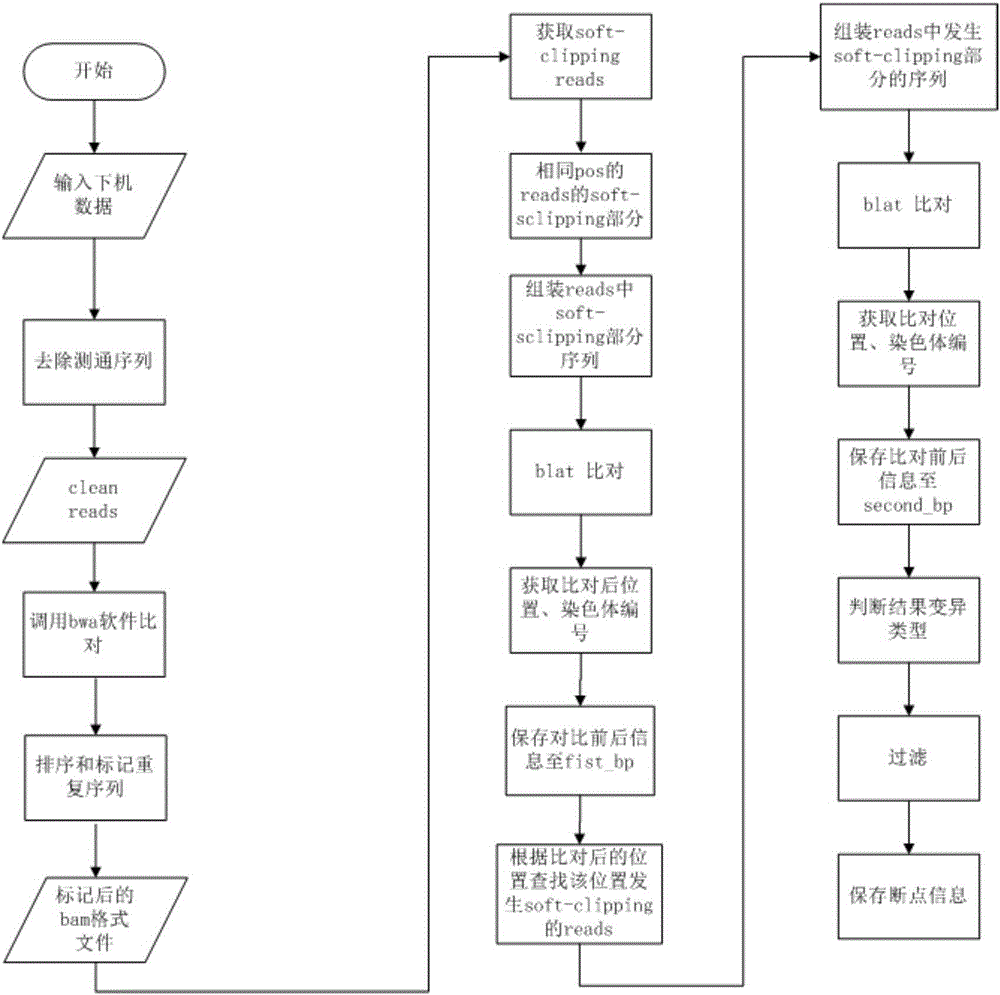
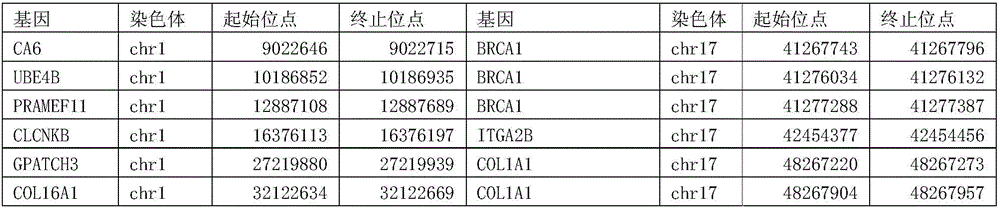
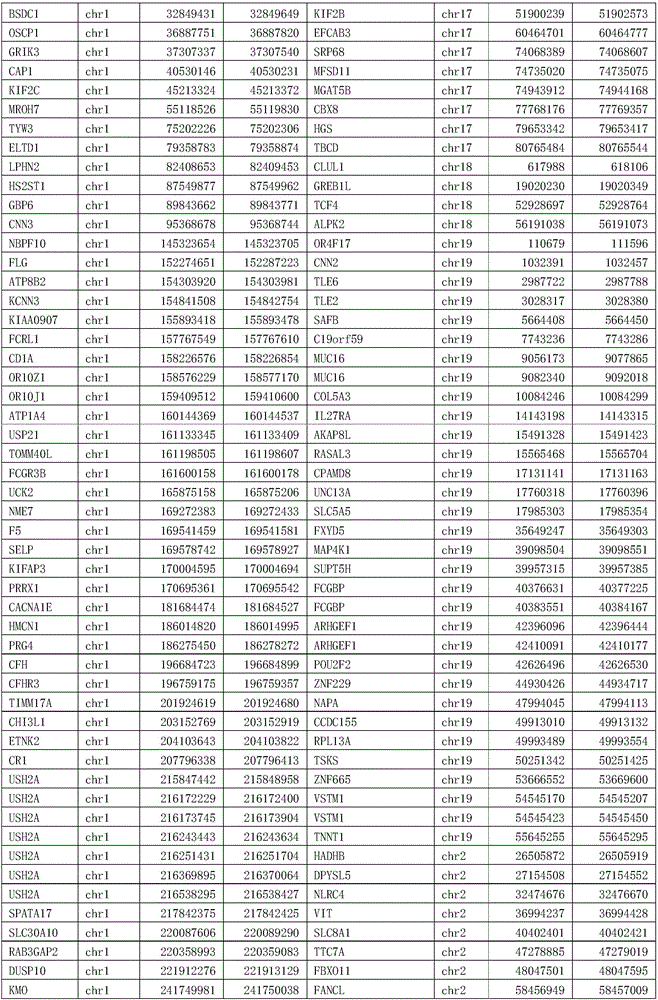
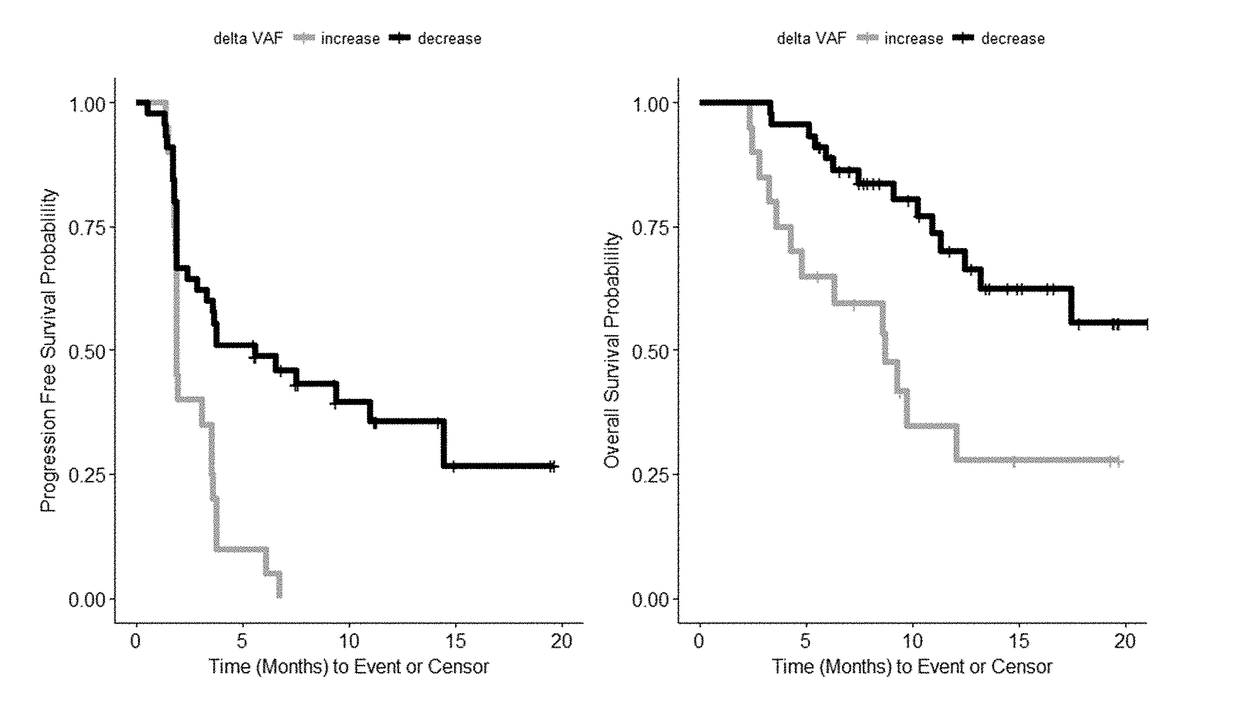
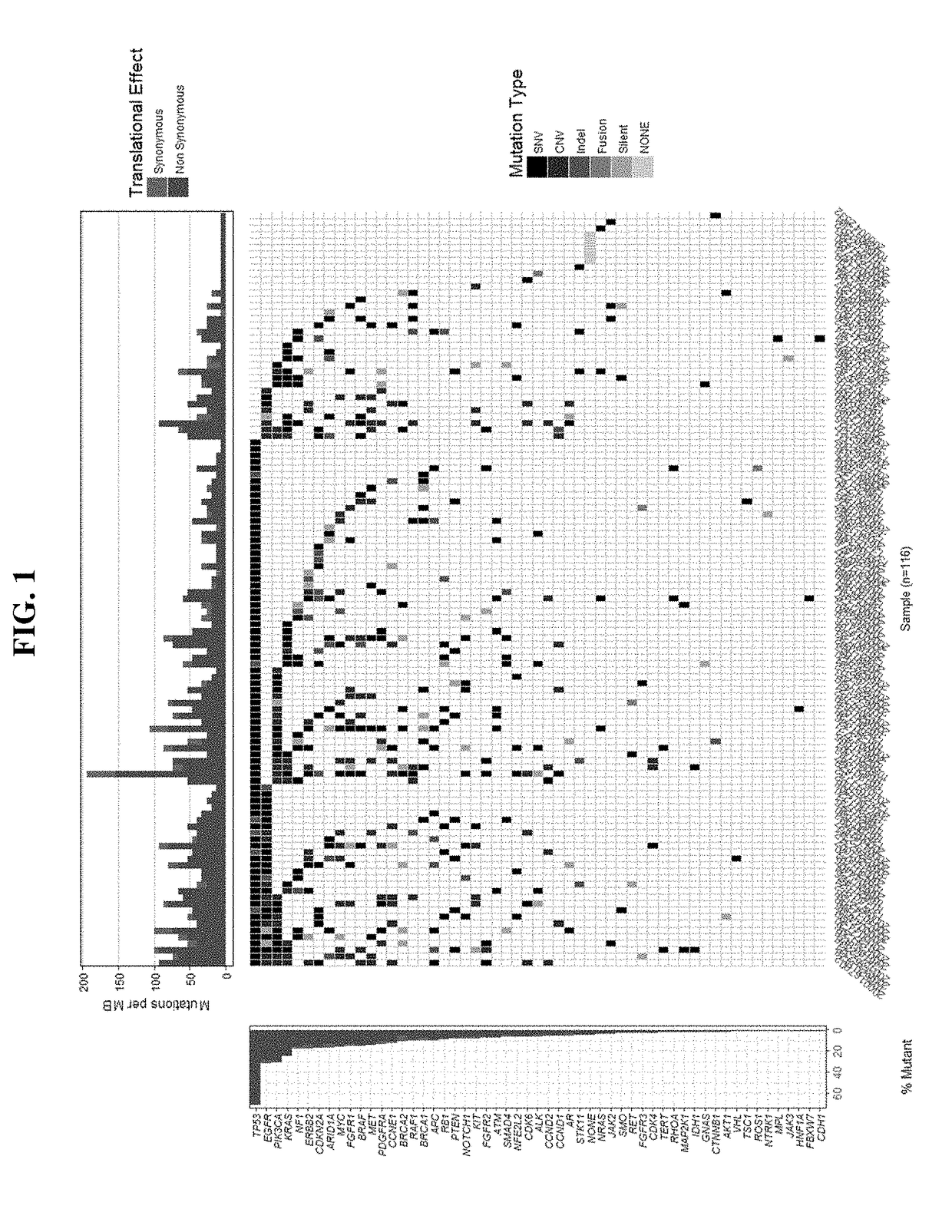
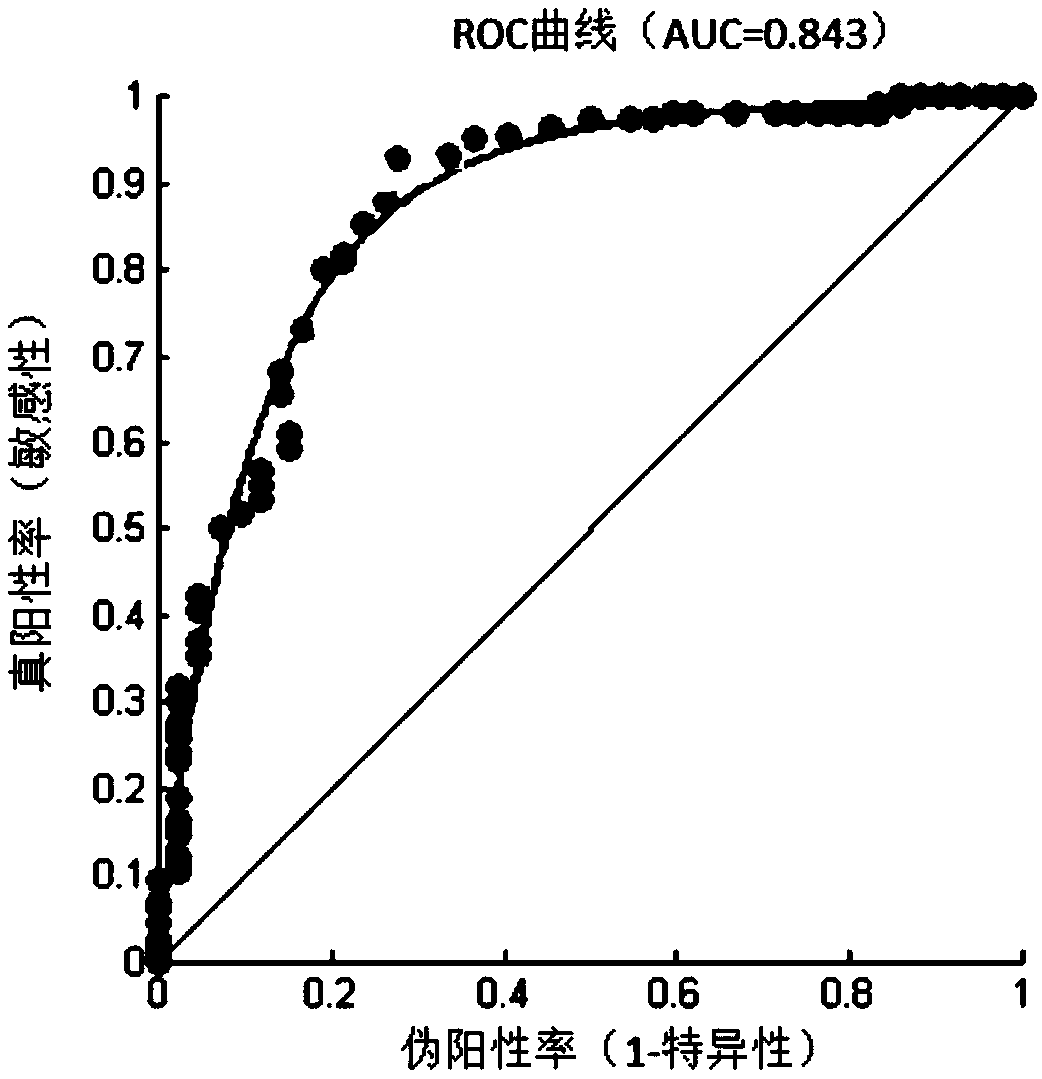
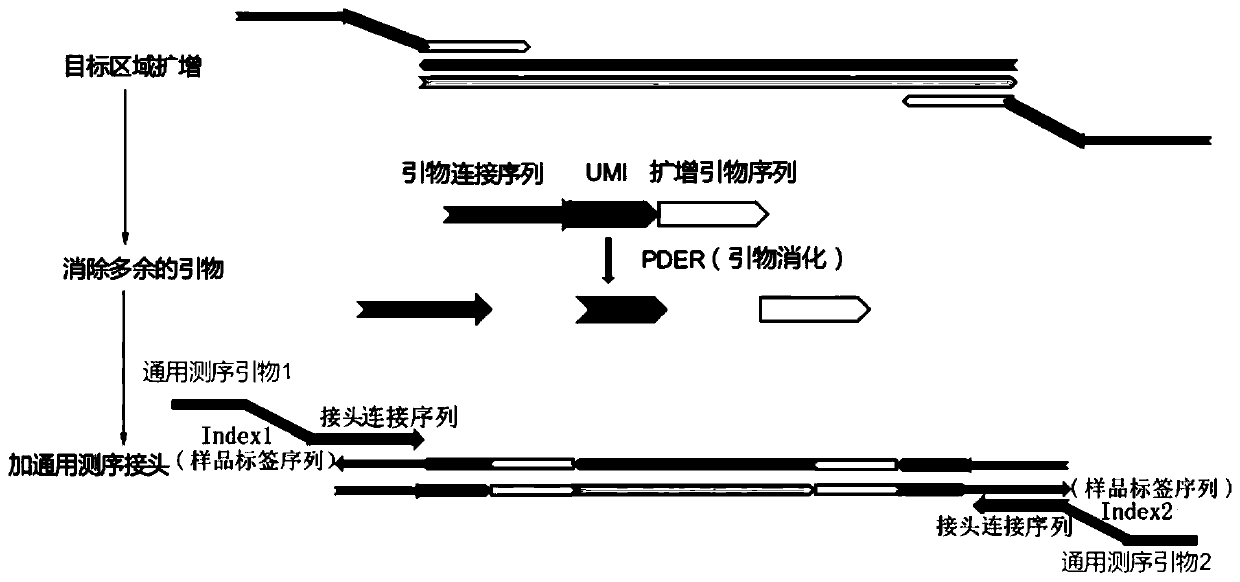
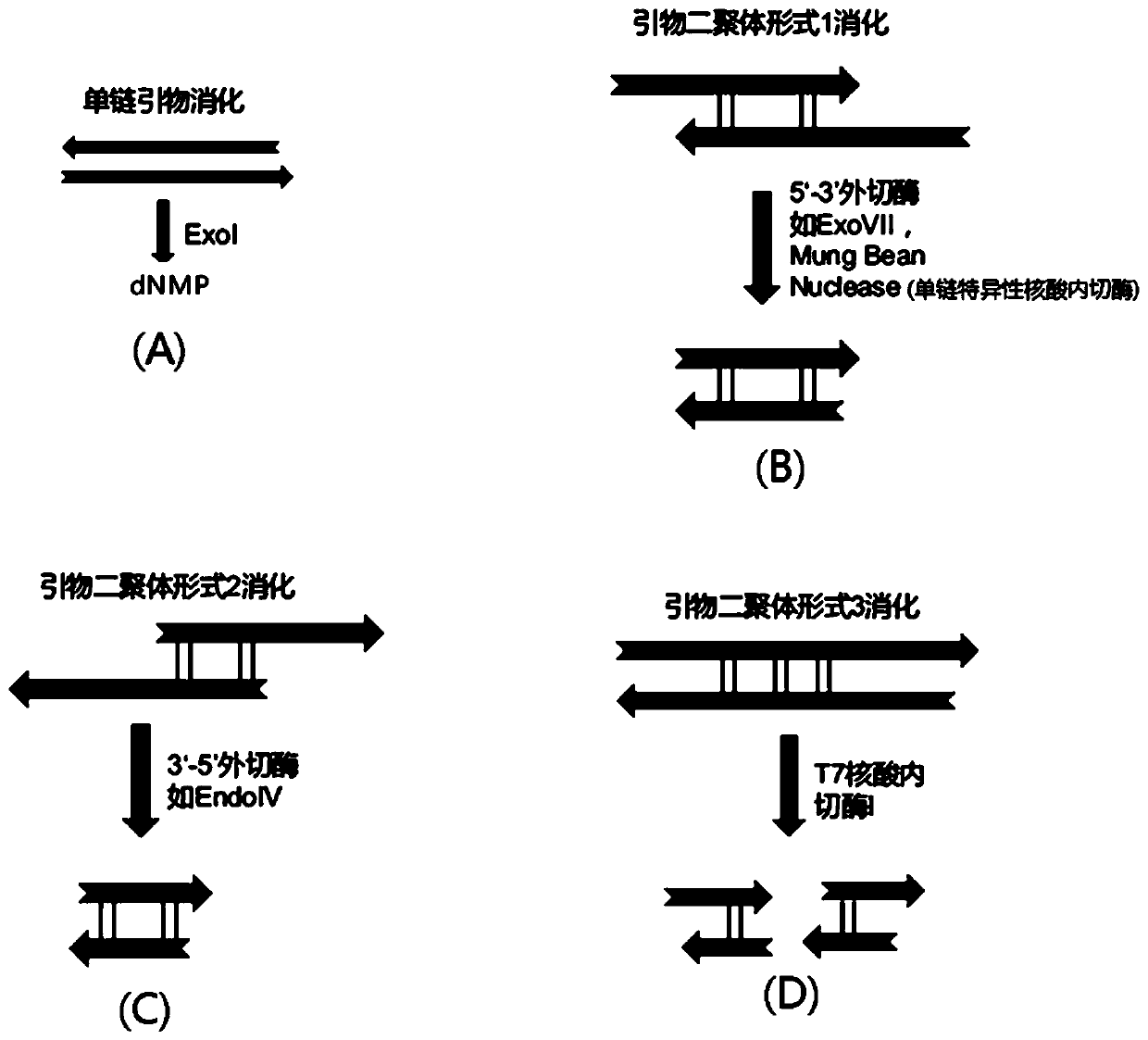
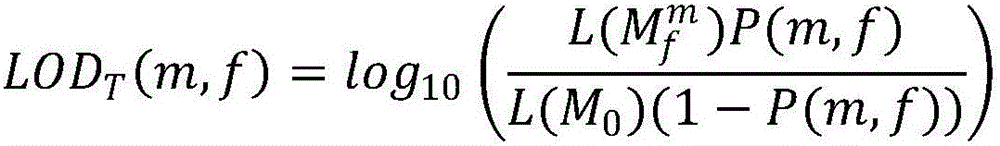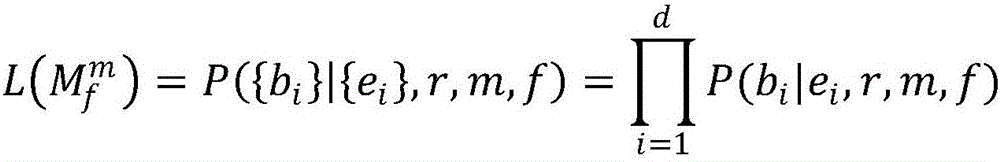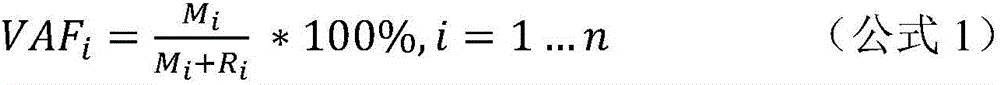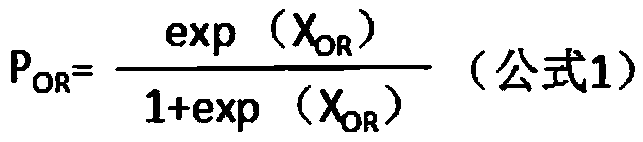Patents
Literature
117 results about "Circulating tumor DNA" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
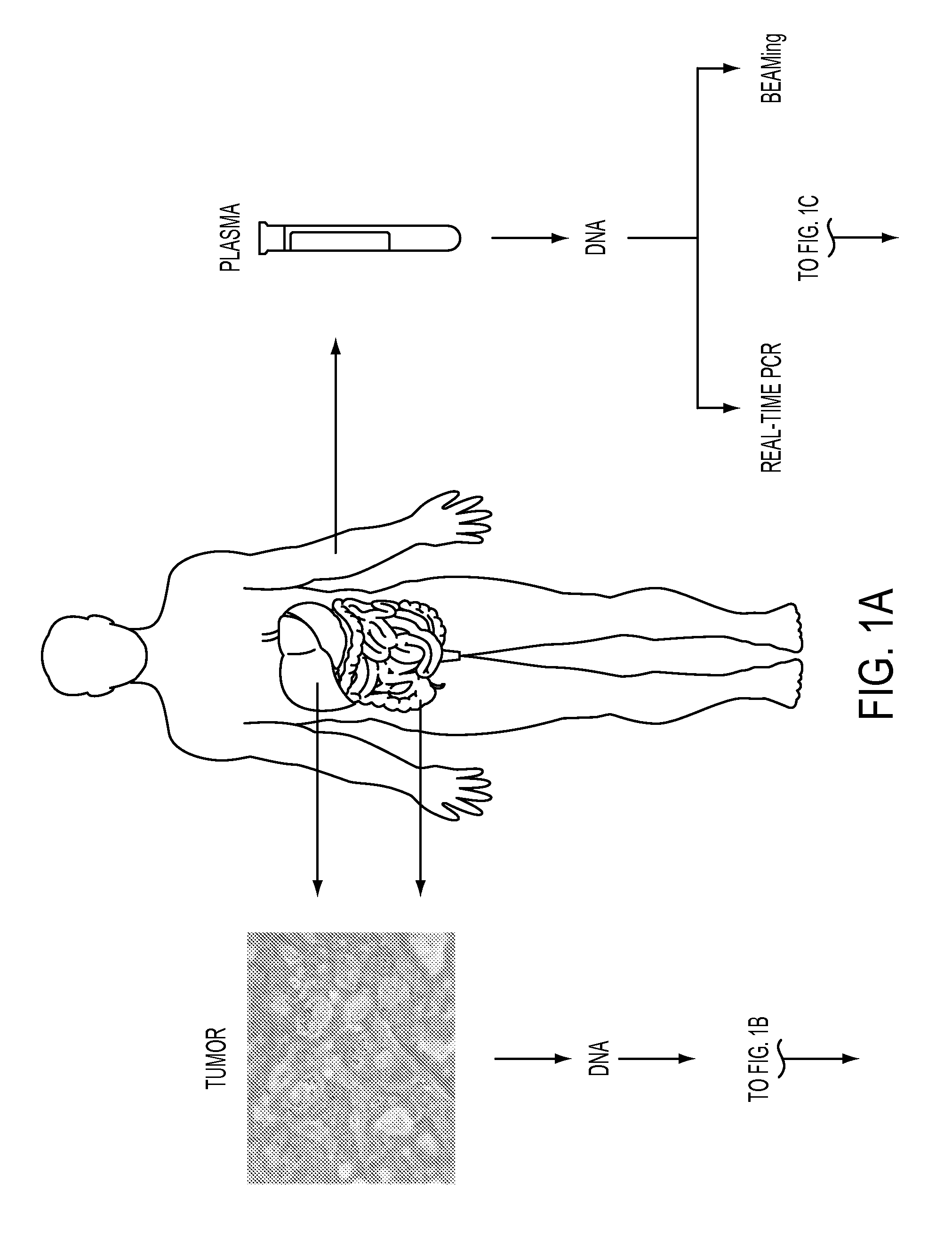
Circulating tumor DNA (ctDNA) is tumor-derived fragmented DNA in the bloodstream that is not associated with cells. ctDNA should not be confused with cell-free DNA (cfDNA), a broader term which describes DNA that is freely circulating in the bloodstream, but is not necessarily of tumor origin. Because ctDNA may reflect the entire tumor genome, it has gained traction for its potential clinical utility; “liquid biopsies” in the form of blood draws may be taken at various time points to monitor tumor progression throughout the treatment regimen.
Circulating Mutant DNA to Assess Tumor Dynamics
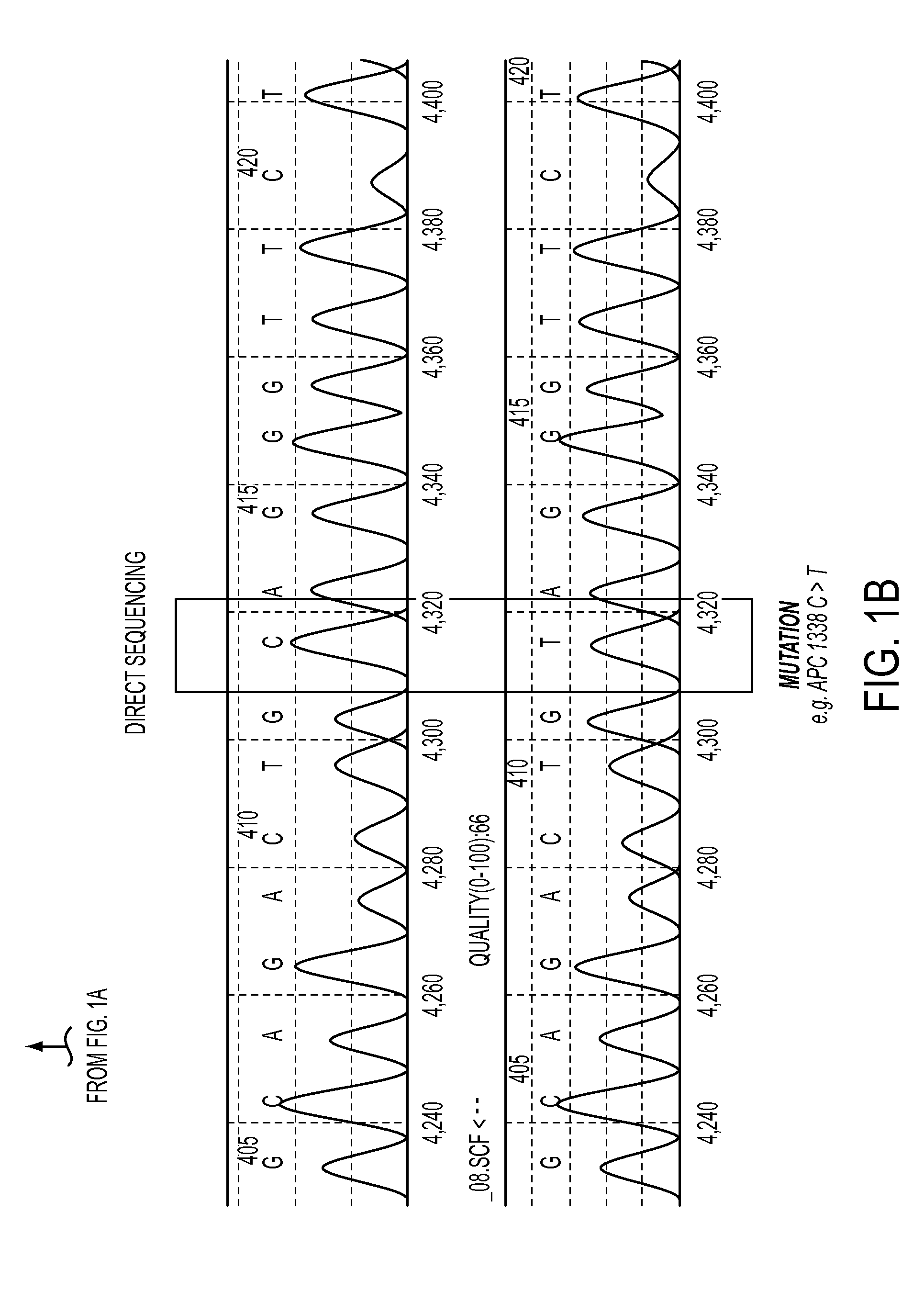
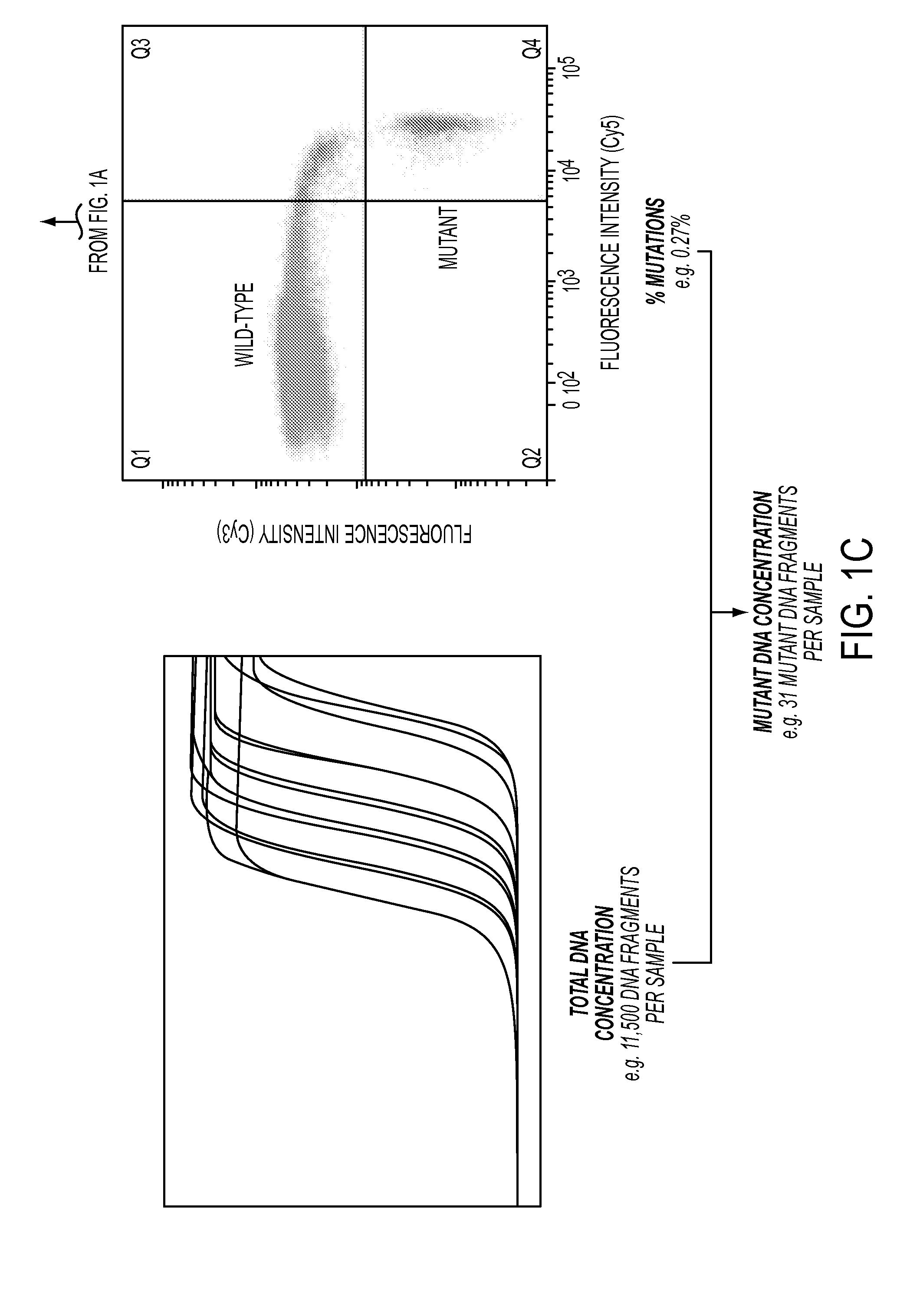
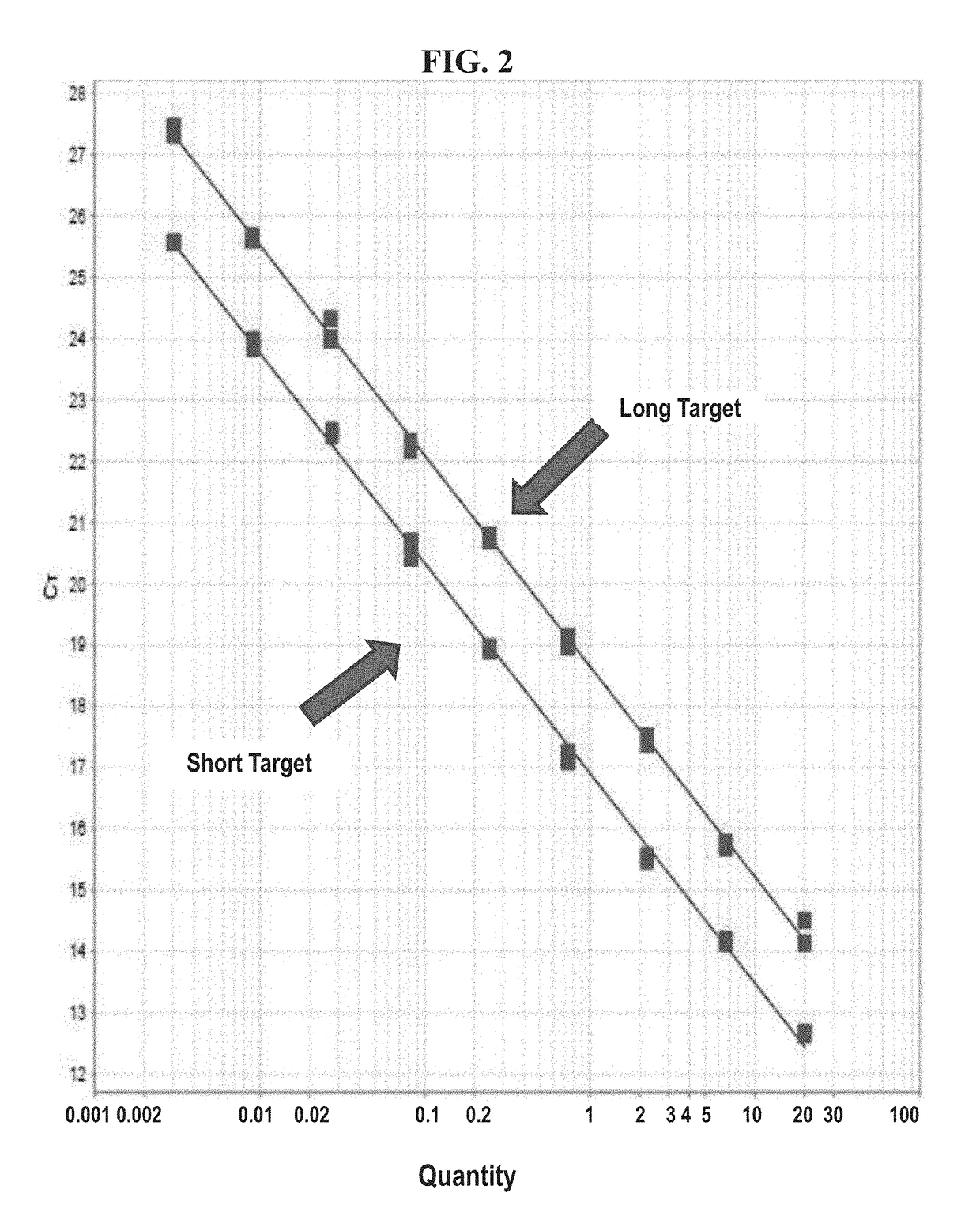
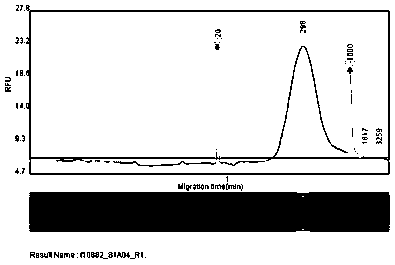
DNA containing somatic mutations is highly tumor specific and thus, in theory, can provide optimum markers. However, the number of circulating mutant gene fragments is small compared to the number of normal circulating DNA fragments, making it difficult to detect and quantify them with the sensitivity required for meaningful clinical use. We apply a highly sensitive approach to quantify circulating tumor DNA (ctDNA) in body samples of patients. Measurements of ctDNA can be used to reliably monitor tumor dynamics in subjects with cancer, especially those who are undergoing surgery or chemotherapy. This personalized genetic approach can be generally applied.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Compositions and methods for detection of nucleic acid mutations
PendingUS20190185913A1Nucleotide librariesMicrobiological testing/measurementCirculating tumor DNAPcr method
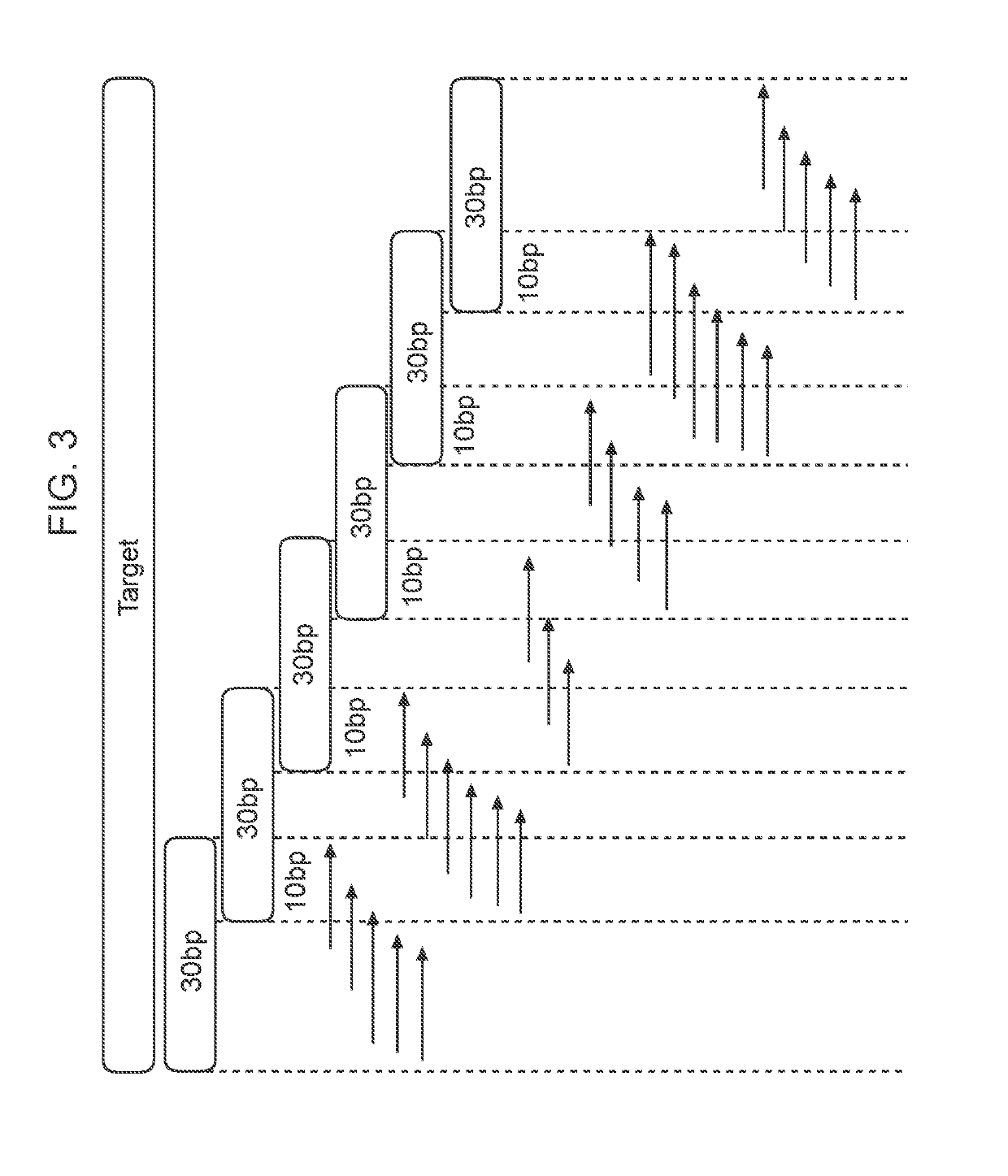
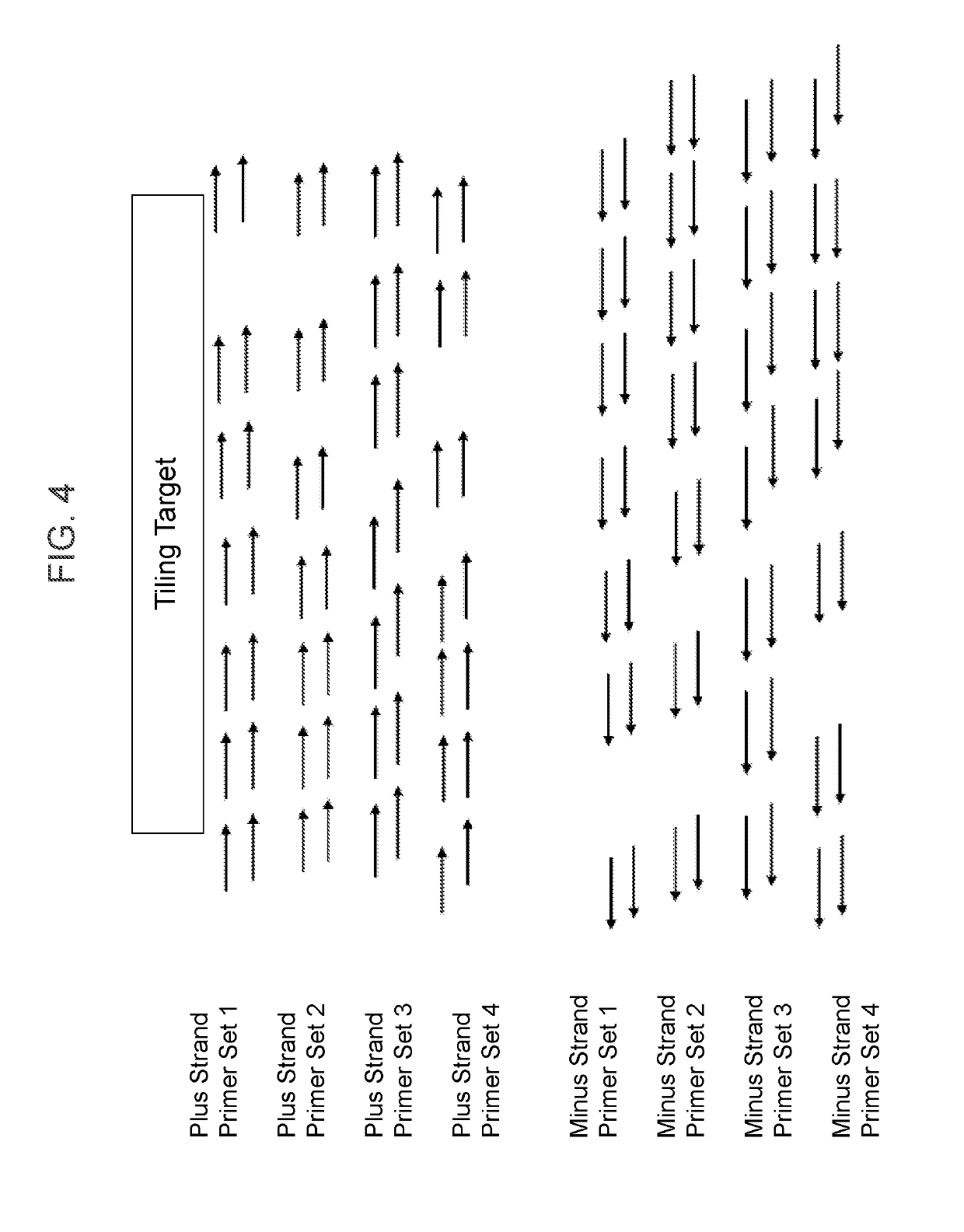
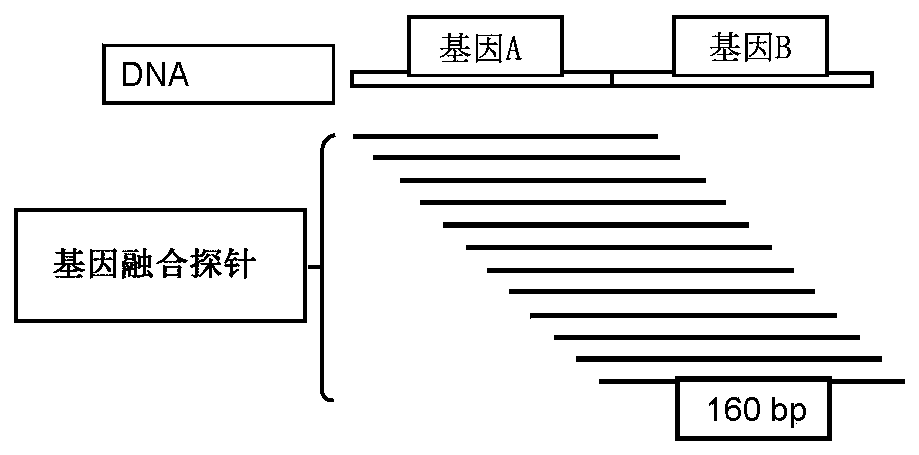
The invention provides methods and compositions for detecting a mutation in a target gene in a sample of blood or a fraction thereof, including in certain examples, a fraction that includes circulating tumor DNA. The methods can include a tiling PCR reaction, for example a one-sided multiplex tiling reaction. Virtually any type of mutation can be detected with the methods and compositions. In certain embodiments, gene fusions are detected. Improved PCR methods, especially for performing nested multiplex PCR reactions are provided.
Owner:NATERA
Method for measuring tumor burden in patient derived xenograft (PDX) mice
ActiveUS20180288982A1Easy to observeMicrobiological testing/measurementScreening processCirculating tumor DNAHuman patient
Kits and methods providing measurement of tumor burden in a patient derived xenograft (PDX) mouse are described. Exemplary embodiments contemplate taking a sample, typically a blood sample, from a PDX mouse and using a real-time polymerase chain reaction (PCR) system to quantitate both human patient circulating tumor DNA (ctDNA) and mouse DNA. In preferred embodiments, both PCR amplifications are done simultaneously in a multiplex, and a highly polymorphic human DNA target sequence is amplified for high sensitivity, allowing for small volume samples, typically 50-100 μL, of mouse blood. Serial evaluations are possible because the mouse can survive withdrawal of these small volumes of blood. A related method allows for quantitation of ctDNA in the presence of human immune cells added to a “humanized” mouse. These relatively quick and easy methods of determining tumor burden in PDX mice can have predictive value for the efficacy of cancer treatments in human patients.
Owner:LIFE GENETICS LAB
Circulating tumor DNA copy number variation detection method and apparatus
ActiveCN108319813ASolve the technical problem of poor accuracy of mutation detectionIncreased variant detection accuracyProteomicsGenomicsCirculating tumor DNACrowds
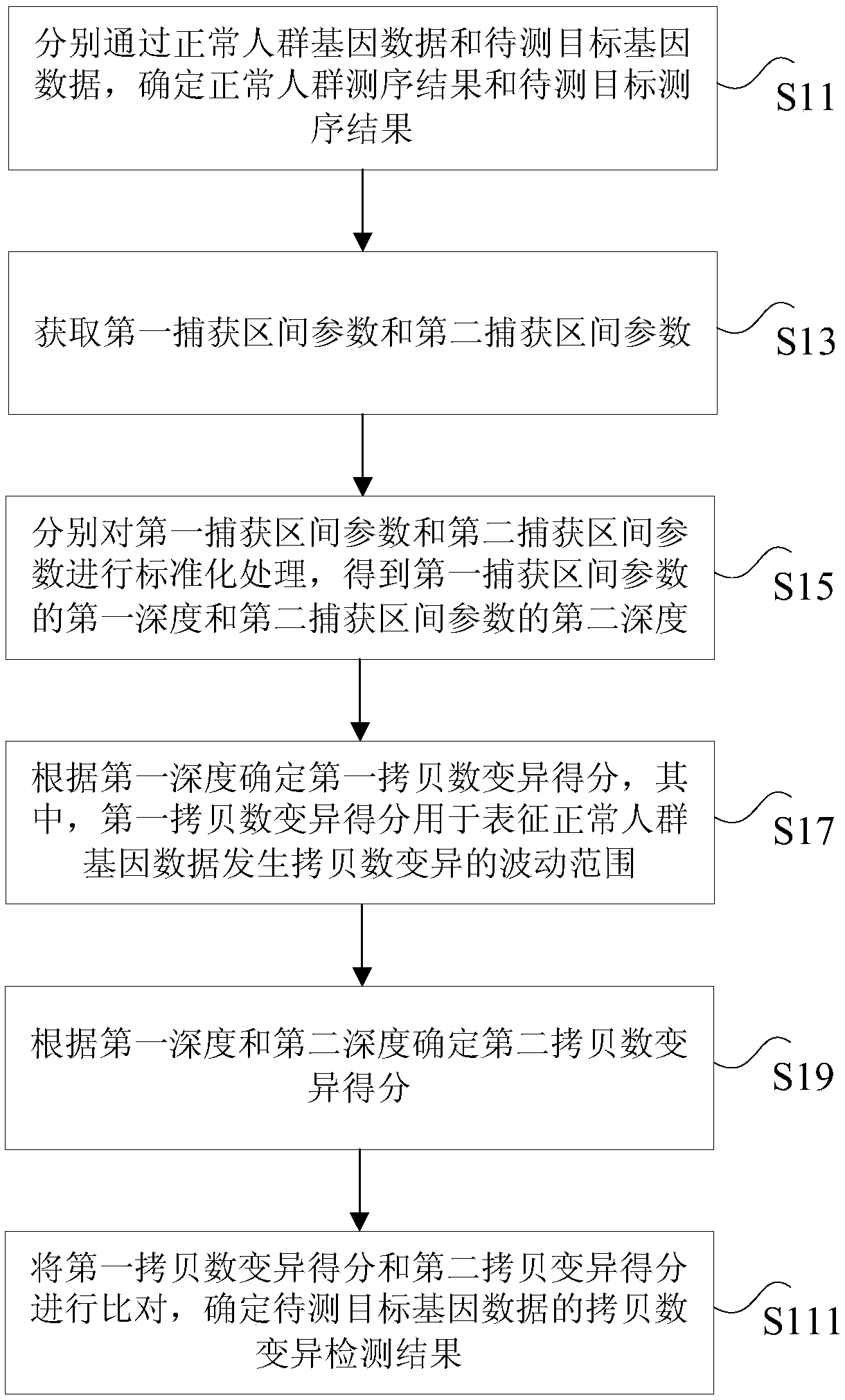
The invention discloses a circulating tumor DNA copy number variation detection method and apparatus. The method comprises the steps of determining a normal crowd sequencing result and a to-be-detected target sequencing result through normal crowd gene data and to-be-detected target gene data; obtaining a first capture interval parameter and a second capture interval parameter; performing standardization processing on the first capture interval parameter and the second capture interval parameter to obtain a first depth of the first capture interval parameter and a second depth of the second capture interval parameter; according to the first depth, determining a first copy number variation score; according to the first depth and the second depth, determining a second copy number variation score; and according to the first copy number variation score and the second copy number variation score, determining a copy number variation detection result of the to-be-detected target gene data. The technical problem of poor circulating tumor DNA copy number variation detection accuracy in the prior art is solved.
Owner:GENE CRAB BIOTECH CO
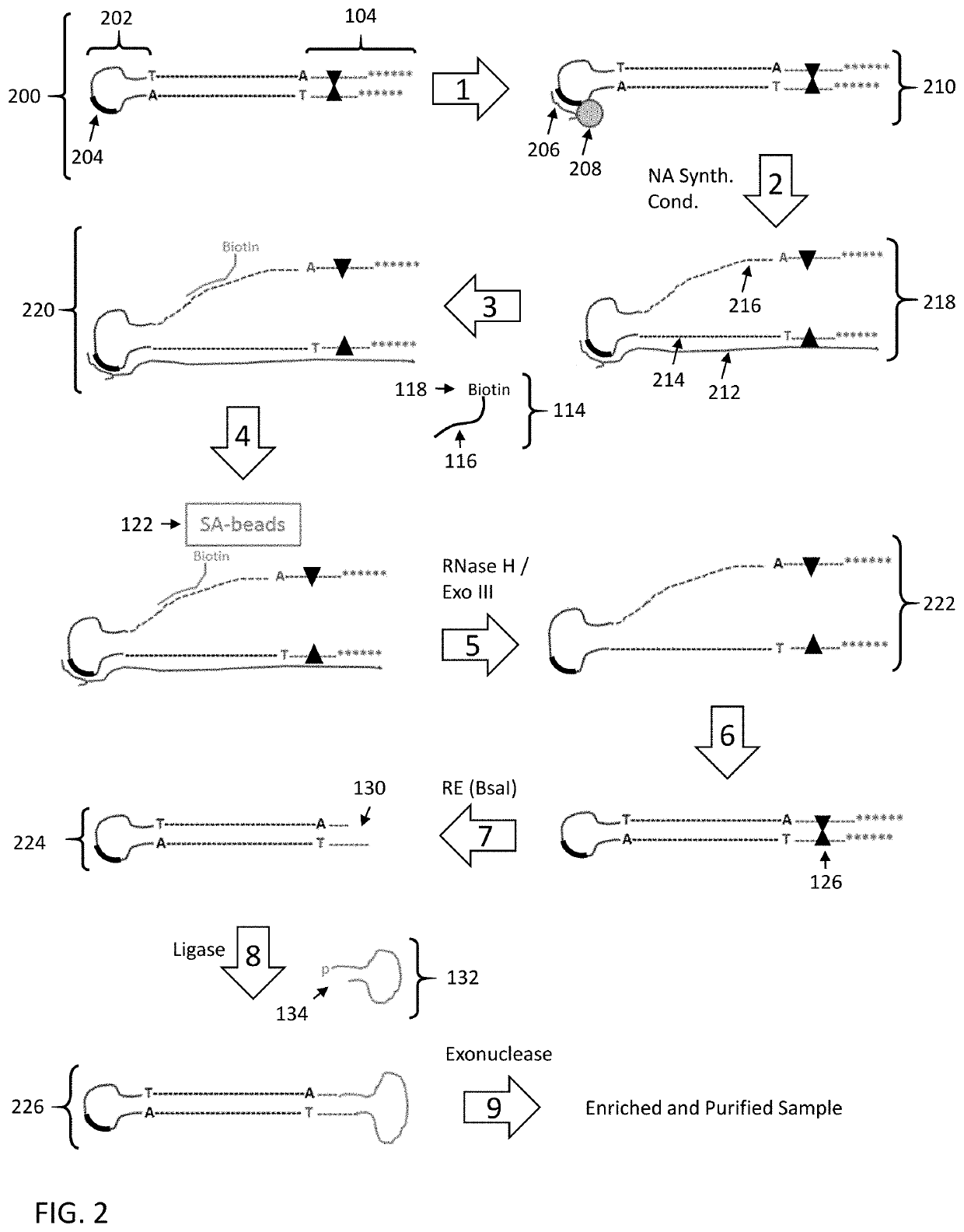
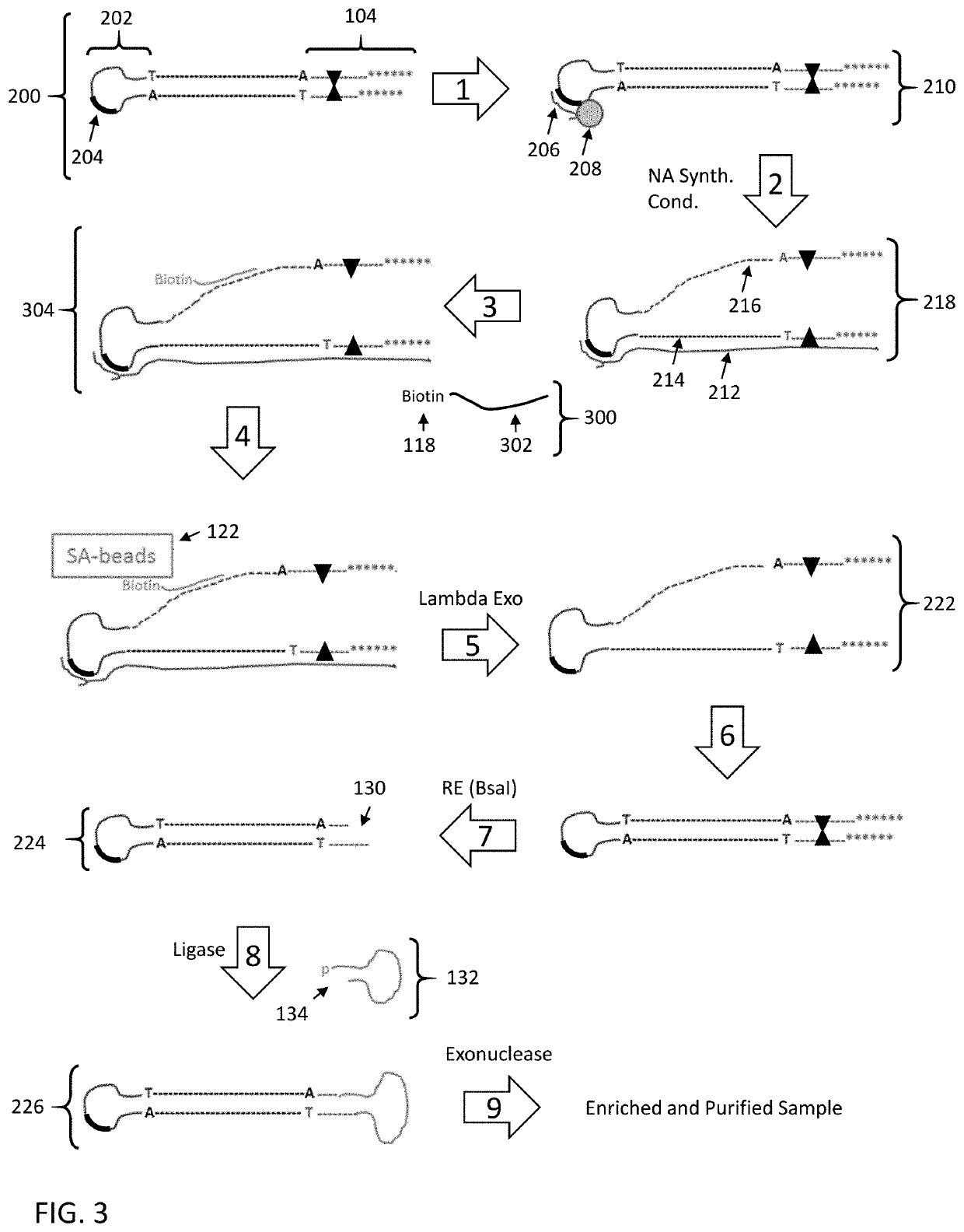
Enrichment of DNA comprising target sequence of interest
PendingUS20190360043A1Nucleotide librariesMicrobiological testing/measurementDownstream processingCell free
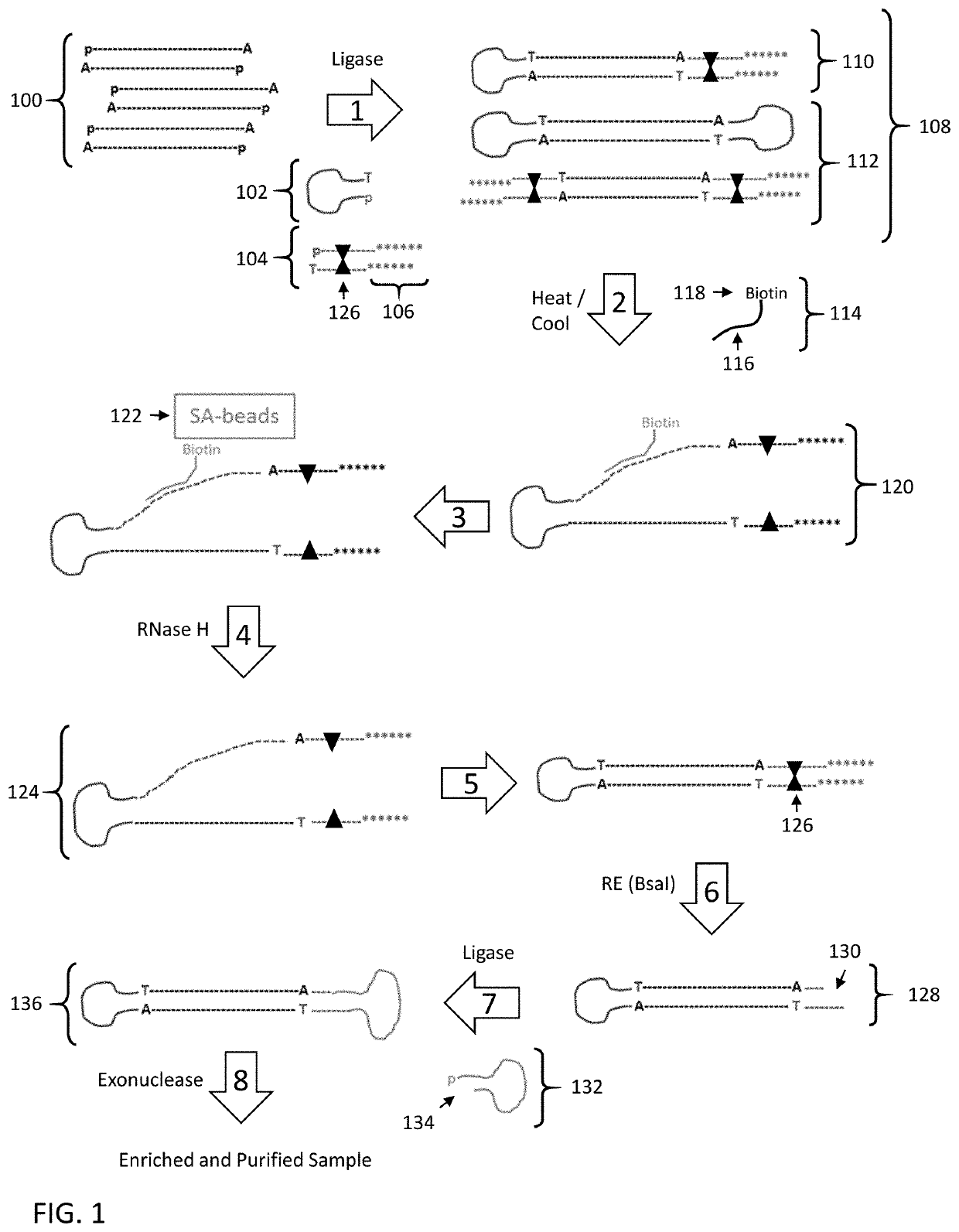
Disclosed are methods and compositions for enriching nucleic acid fragments from a sample that include one or more target region of interest. In certain aspects, a sample of double stranded nucleic acid fragments having a strand-linking adapter at one end and a non-strand-linking adapter at the other end are denatured and contacted with capture probes specific for a target sequence of interest. Capture probe-bound fragments are isolated from the sample, e.g., using a solid substrate specific for the binding moiety on the capture probes, and are renatured for downstream processing, thus maintaining the original double-stranded region. This enrichment process does not require amplification and as such maintains the nucleic acids in their native states. The disclosed enrichment process and compositions are suitable for analyzing nucleic acids that are fragmented and / or damaged, e.g., cell-free DNA such as circulating tumor DNA, as well as nucleic acids that are many kilobases in length.
Owner:PACIFIC BIOSCIENCES
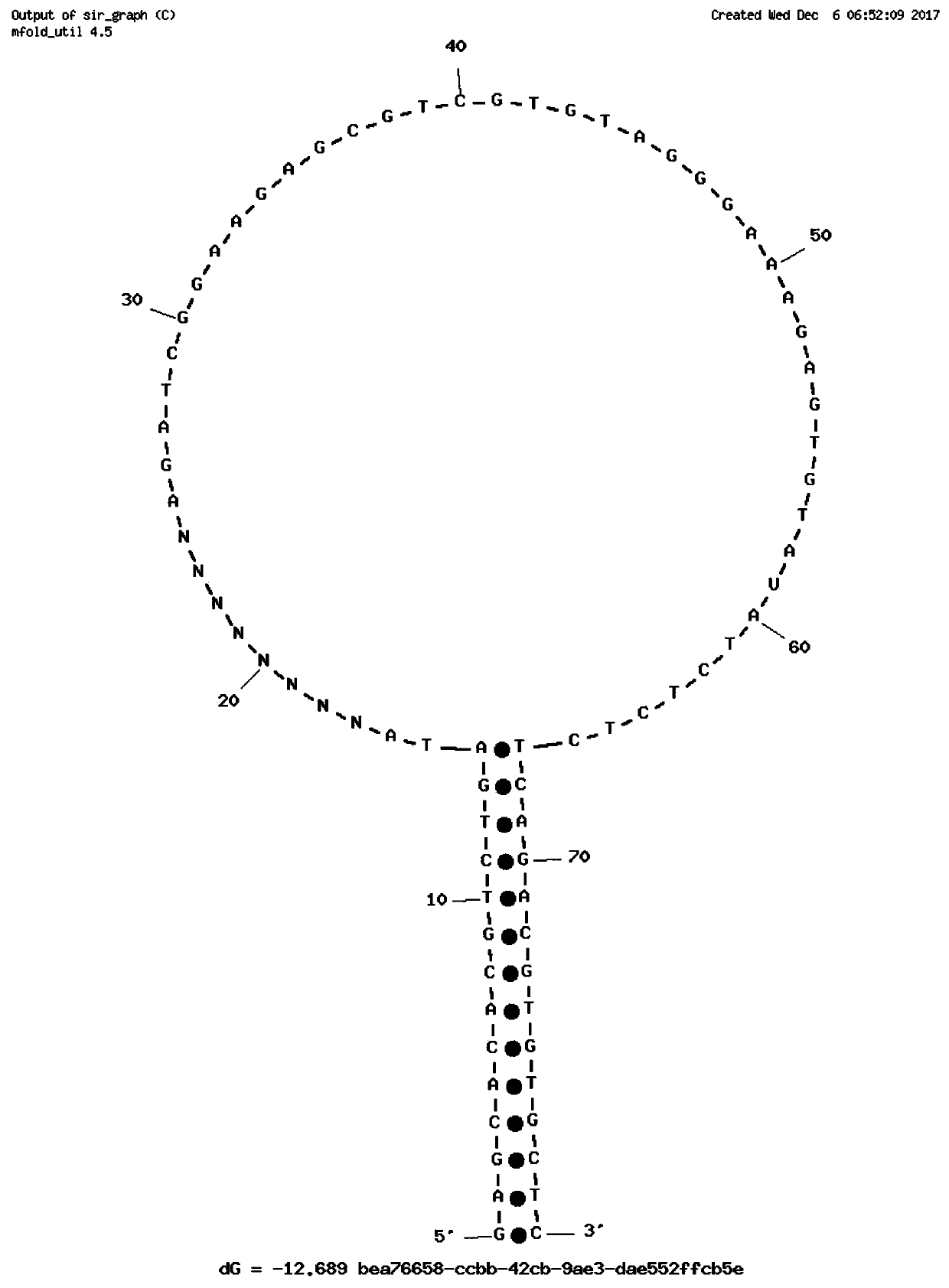
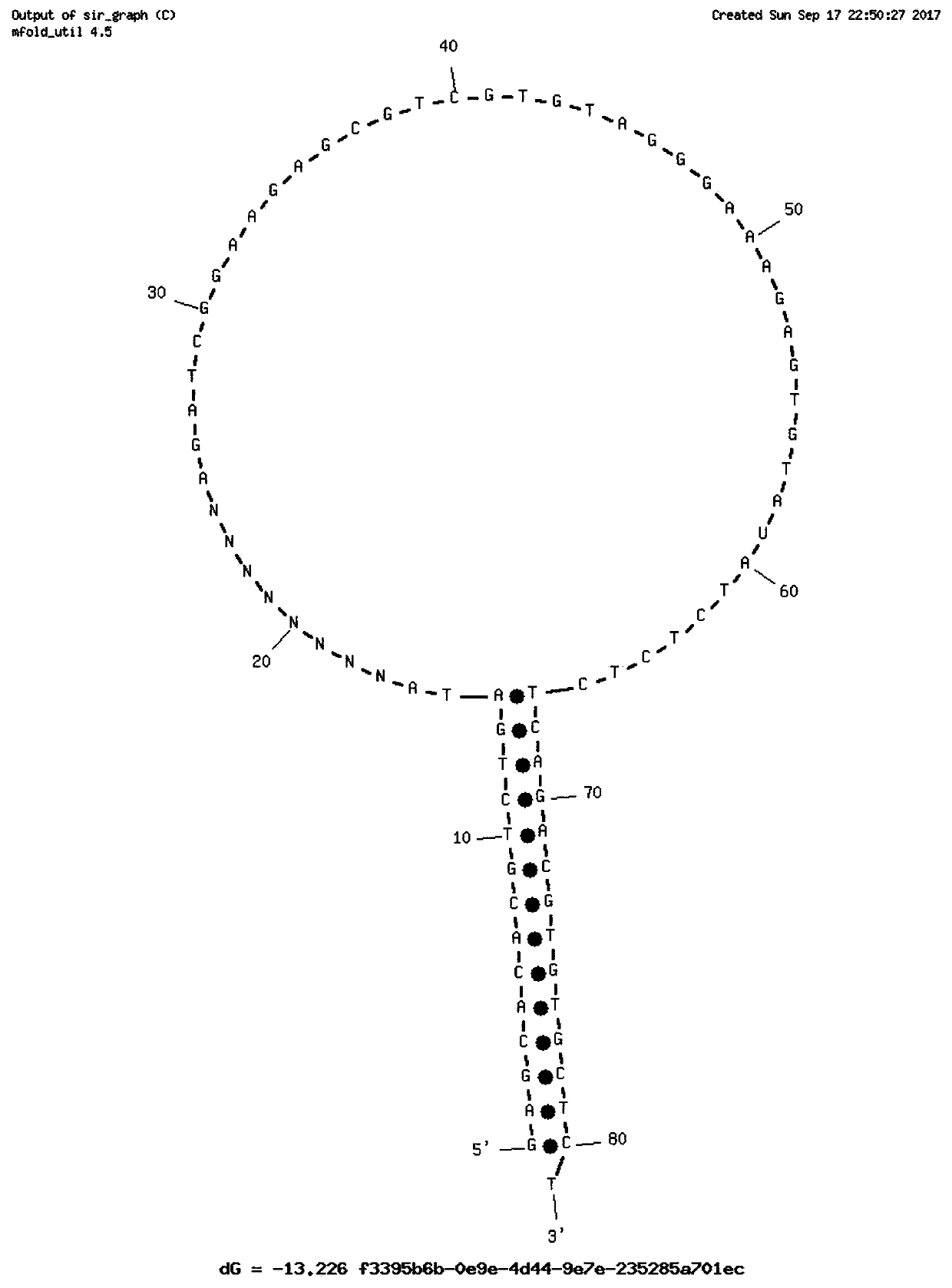
Single-chain molecular identifier adapter and single-chain DNA database creating method and application thereof to circulating tumor DNA detection
InactiveCN109797197AReduce dosageHigh-resolutionMicrobiological testing/measurementLibrary linkersDNA databaseSingle strand dna
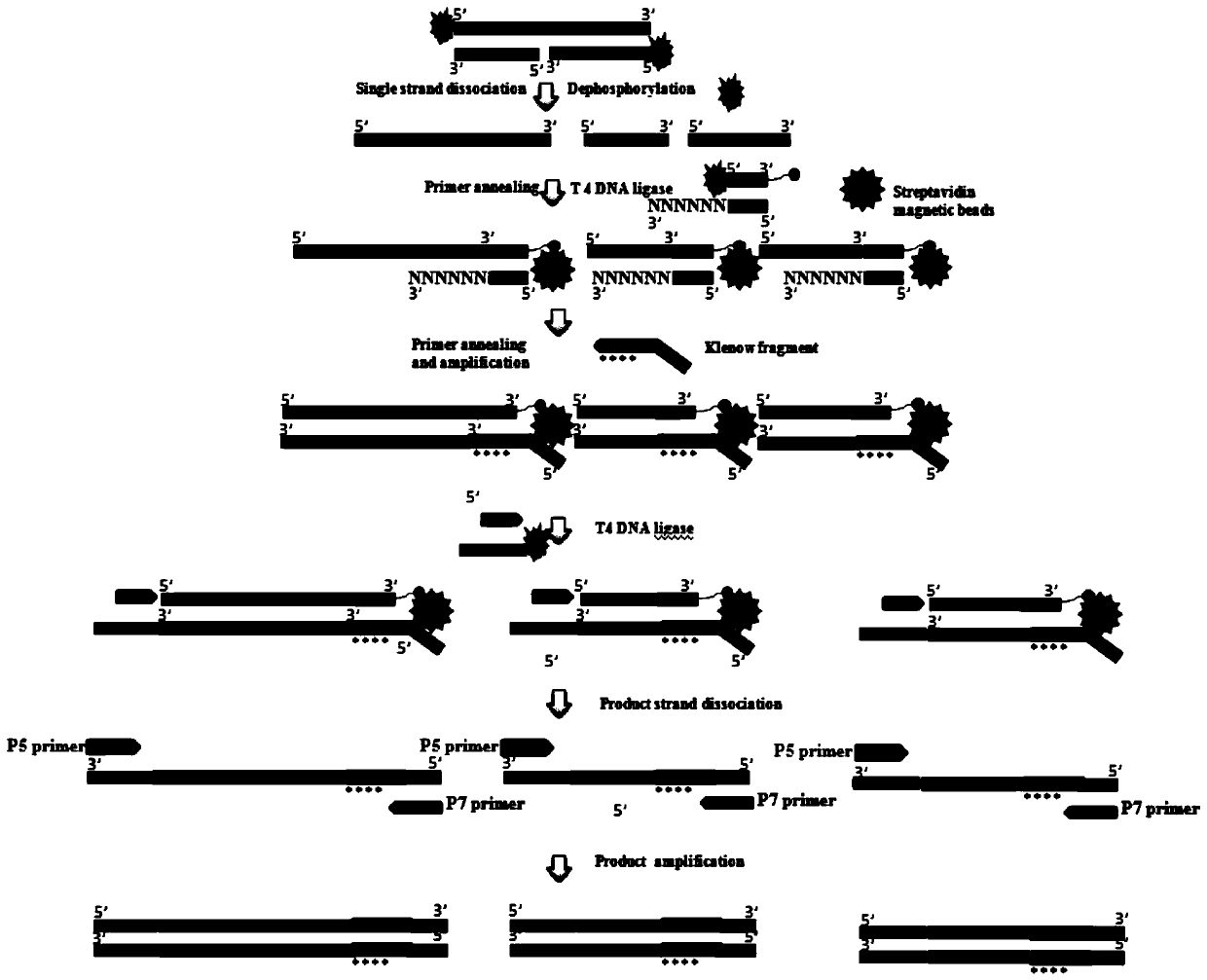
The invention discloses a single-chain molecular identifier adapter and a single-chain DNA database creating method and application thereof to circulating tumor DNA detection. The single-chain molecular identifier adapter comprises a stem structure formed by 14 base pairing, a sequencing primer sequence and a molecular identifier sequence formed by 8 random nucleotides, wherein a base 'U' is inserted between the sequencing primer sequence and a stem structure sequence, and a stem-loop structure can be formed by annealing denaturation of the single-chain nucleotide sequence. By single-chain DNAdatabase creating, technical problems of low sensitivity, fragmentation, low concentration and short ctDNA fragments in liquid biopsy in application of an existing detection technique are solved. Accurately distinguishing whether sequencing reads identical in genome coordinate beginning and end loci come from cfDNA released by the same one or multiple germinal cell is realized, sequencing reads derived from original positive and negative chains of the same cell are paired for analysis, and accordingly sequencing rehandling is reduced, and the distinguishing rate of false positive mutation isincreased.
Owner:HANGZHOU NEOANTIGEN THERAPEUTICS CO LTD
Biological information processing method for analyzing circulating tumor DNA (deoxyribonucleic acid)
InactiveCN107523563ASample collection is simple and convenientKeep valid dataMicrobiological testing/measurementHybridisationData setData quality
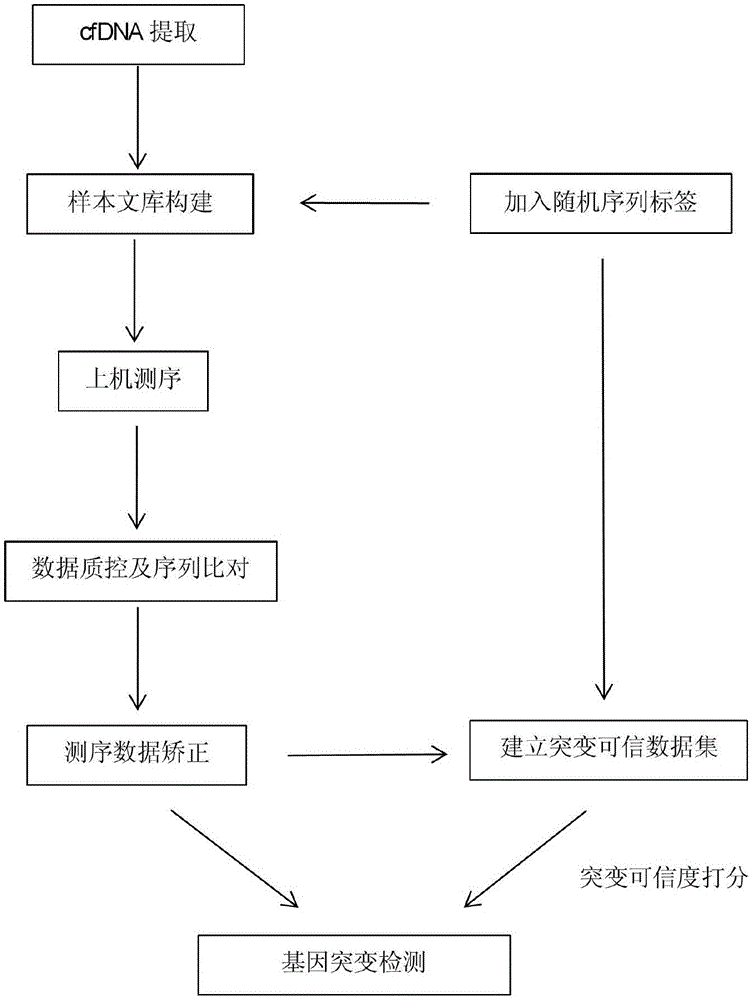
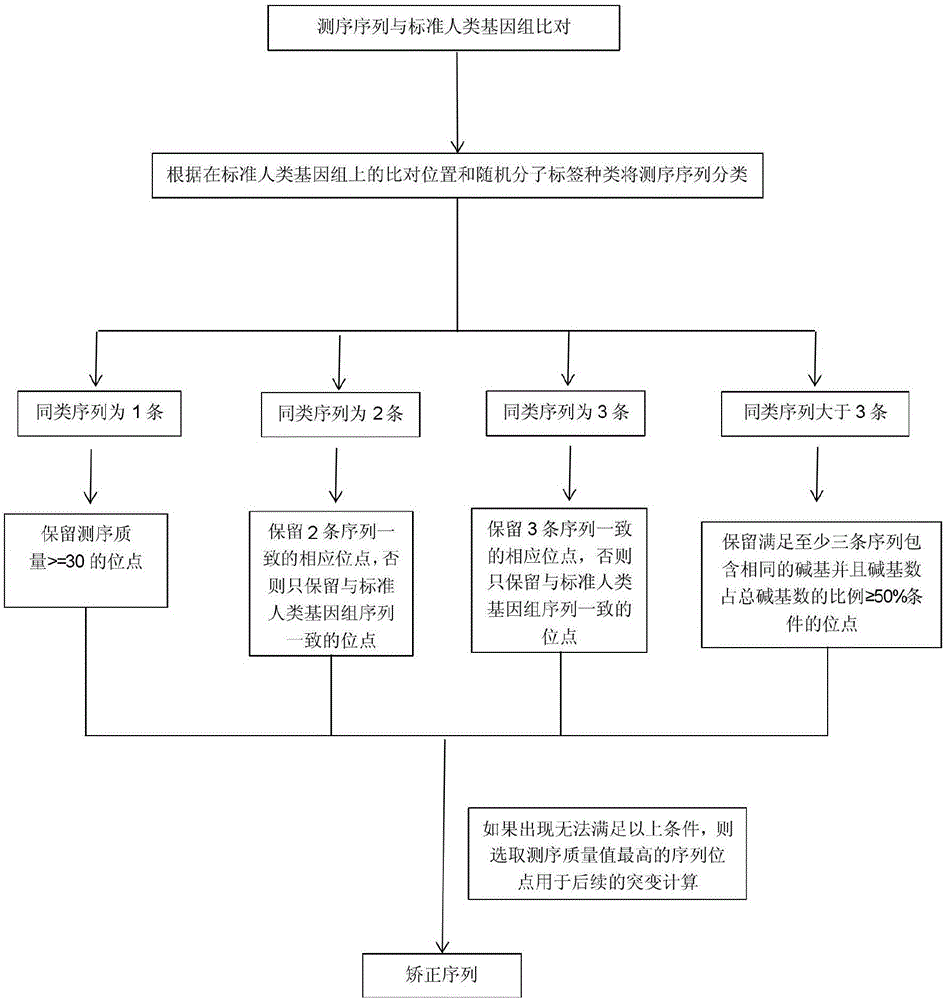
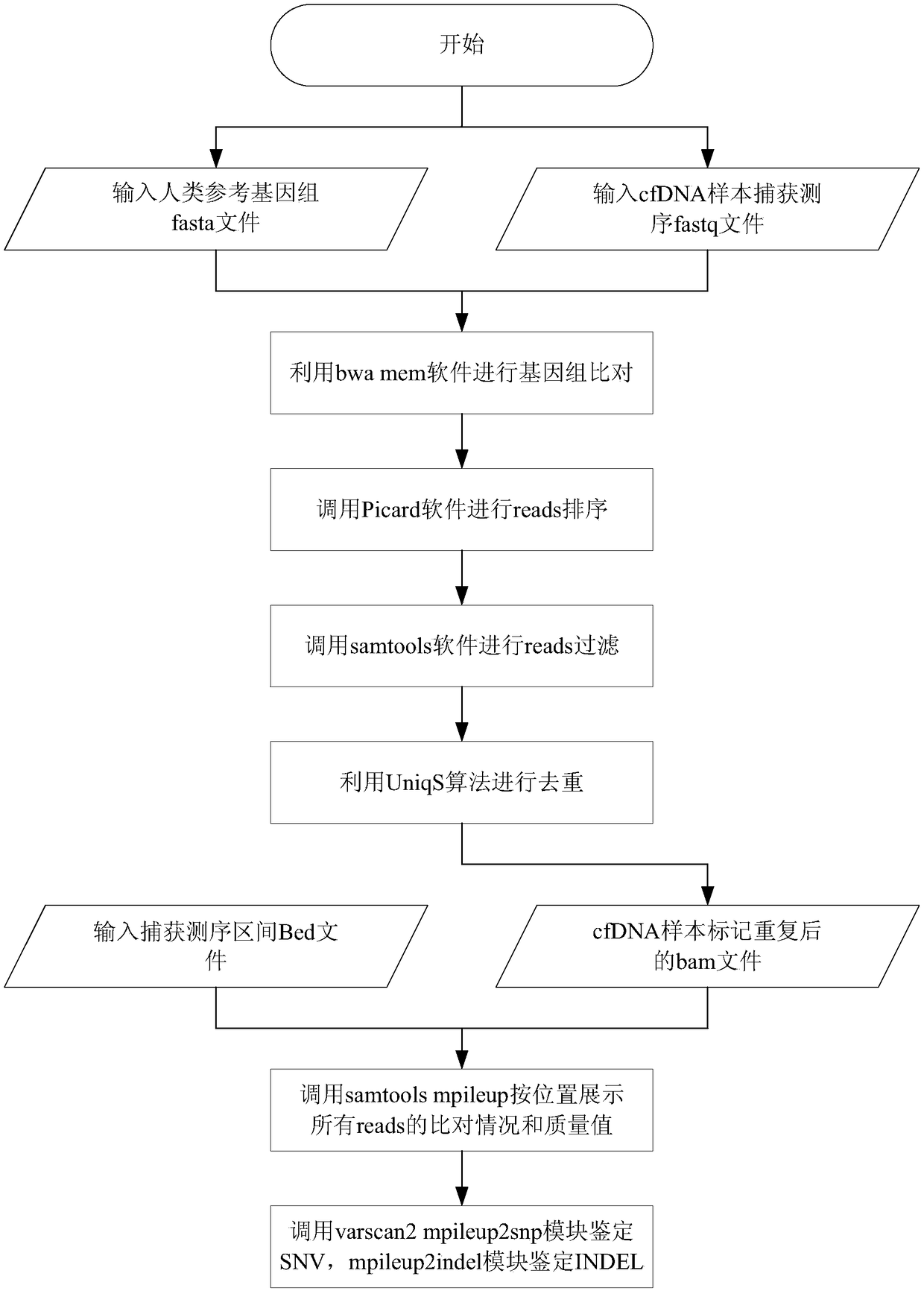
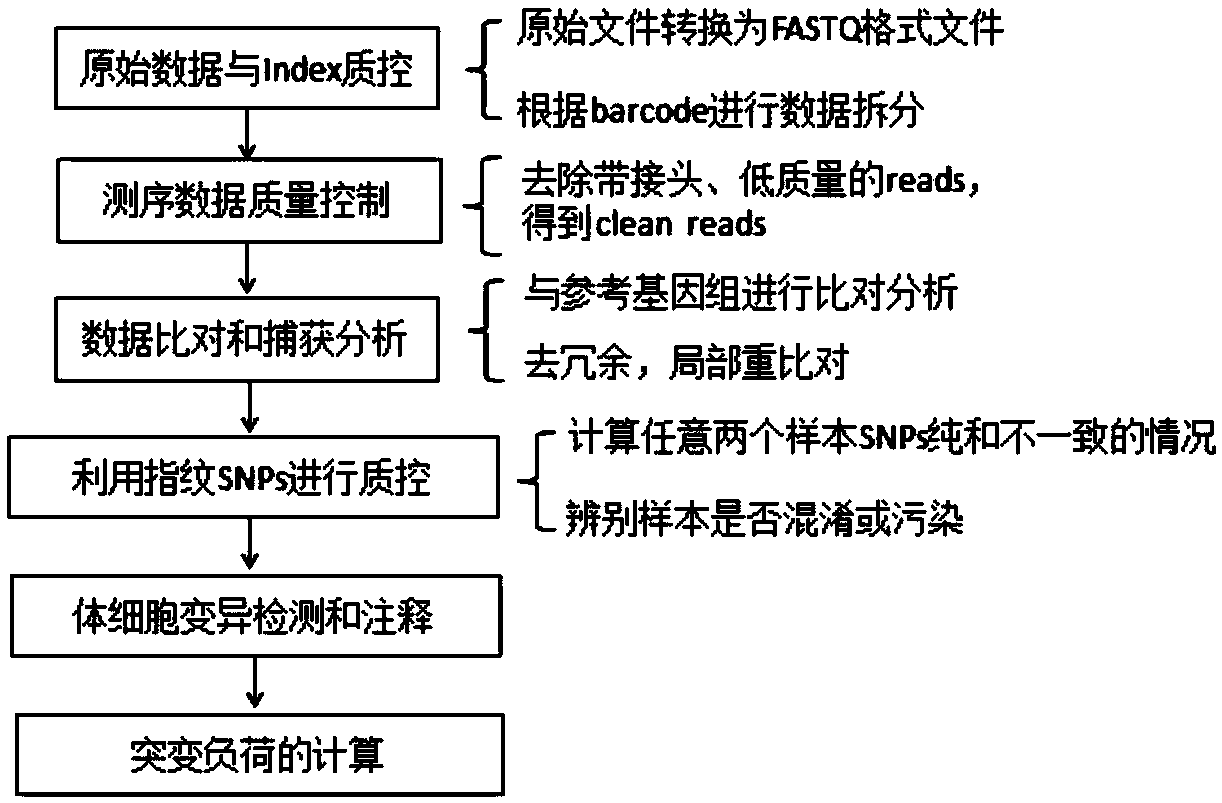
The invention provides a biological information processing method for analyzing circulating tumor DNA (deoxyribonucleic acid). The method includes 1), performing cfDNA extraction, database creating and sequencing; 2), performing sequencing-data quality control and sequencing comparison; 3), performing sequencing data correction; 4), using two pieces of software to simultaneously perform gene mutation detection on the sequencing data corrected in the step 3) and summing and integrating analysis results of the software; 5), establishing a mutant trusted data set by the aid of a sequence corrected in the step 3) and using the data set to provide credibility support to a mutation result acquired in the step 4). The ctDNA in the cfDNA is taken as a detection object, detection can be performed by only collecting a small amount of peripheral blood of a subject, and conciseness and convenience in sample collection are achieved.
Owner:HANGZHOU HEYI GENE TECH
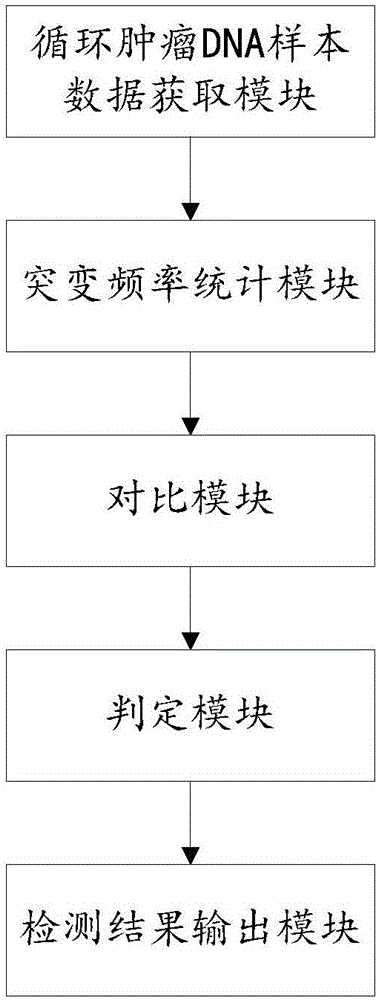
Device for detecting gene fusion of circulating tumor DNA (deoxyribonucleic acid) samples
ActiveCN106845150AReduce the number of comparisonsImprove stabilitySequence analysisHybridisationSequencing dataCirculating tumor DNA
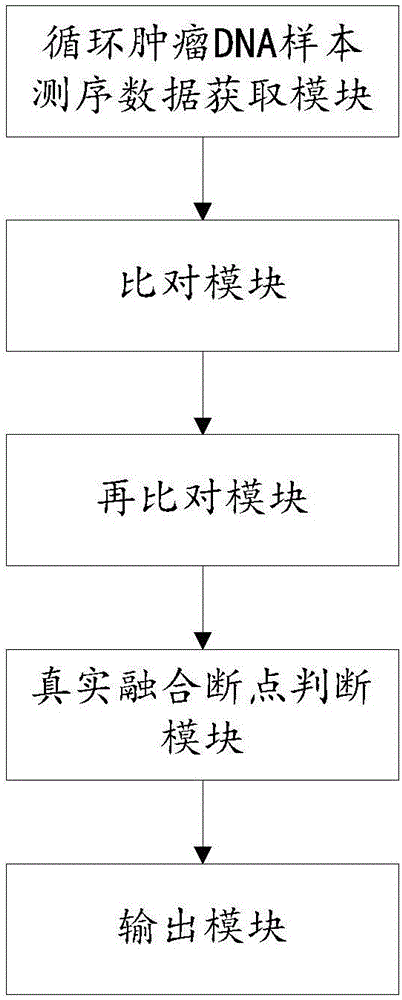
The invention relates to a device for detecting gene fusion of circulating tumor DNA (deoxyribonucleic acid) samples. The device comprises a sequencing data acquisition module, a comparison module, a re-comparison module, a real fusion breakpoint judgment module and an output module. The device for detecting the gene fusion of the circulating tumor DNA samples has the advantages of high detection speed and stability and low resource requirement.
Owner:ZHEJIANG ANNOROAD BIO TECH CO LTD +2
Library for performing high-throughput detection on circulating tumor DNA target genes, as well as detection method and application of library
InactiveCN107447258AImprove detection efficiencyReduce testing costsMicrobiological testing/measurementLibrary creationNon invasiveExon
The invention relates to the field of genomic high-throughput sequencing, in particular to a library for performing high-throughput detection on circulating tumor DNA target genes, as well as a detection method and application of the library. Circulating body fluid of a tumor patient is used as a biological sample, low-concentration free nucleic acid is extracted, the low-concentration free nucleic acid of the biological sample is used as a sample template, target gene exons of the sample template are amplified in a multiplex PCR form, then the tail ends of obtained amplicons are repaired and the 3' ends of the amplicons are connected with A basic groups, the modified amplicons are connected to Illumina sequencing adapters, and then library PCR is carried out by using an index-containing primer, and an amplified product is the library for detecting mutation diversity of the circulating tumor DNA target genes. The in-vitro circulating body fluid of the tumor patient is used as the biological sample for achieving non-invasive detection; the low-concentration free nucleic acid is used as the template for highly-sensitive detection; multiple target gene exons are amplified in a multiplex PCR form at the same time so as to improve the detection efficiency; via establishment of an amplicon library, high-throughput sequencing and data analysis, the detection cost can be greatly reduced.
Owner:DALIAN MEDICAL UNIVERSITY
Device for detecting somatic cell mutation by circulating tumor DNA (deoxyribonucleic acid) samples
PendingCN106845153AAccurate distinctionHigh sensitivitySequence analysisSpecial data processing applicationsMutation frequencyCirculating tumor DNA
The invention relates to a device for detecting somatic cell mutation by circulating tumor DNA (deoxyribonucleic acid) samples. The device comprises a data acquisition module, a mutation frequency statistics module, a comparison module, a judgment module and a detection result output module. The device for detecting the somatic cell mutation by the aid of the circulating tumor DNA samples has the advantages that system errors can be accurately differentiated from the real somatic cell mutation by the device, accordingly, the sensitivity can be improved, and false positive and false negative can be reduced.
Owner:ANNOROAD GENE TECH BEIJING +2
Circulating tumor DNA EGFR detecting technology and reagent kit thereof
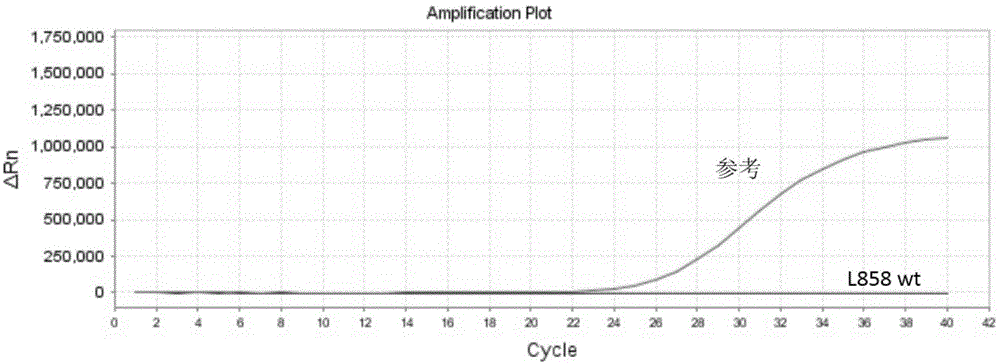
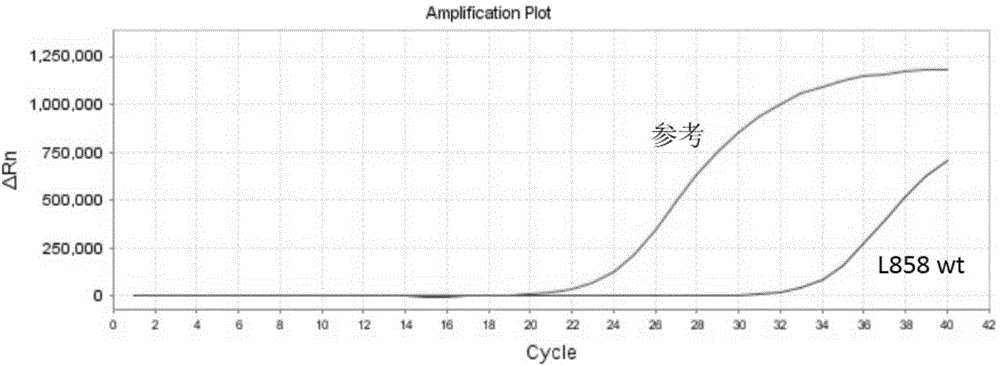
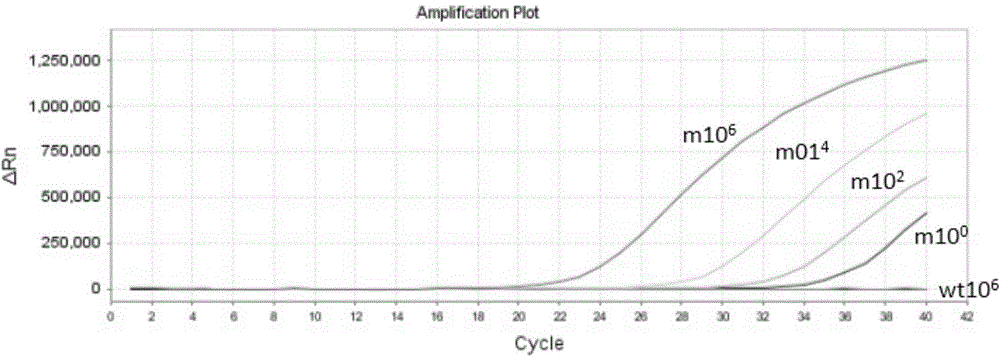
InactiveCN104789677AHigh sensitivityEasy to operateMicrobiological testing/measurementWild typeCirculating tumor DNA
The invention discloses a circulating tumor DNA EGFR (Epidermal Growth Factor Receptor) detecting technology and a reagent kit thereof. The circulating tumor DNA EGFR detecting technology comprises the following steps: step 1, designing a synthetic primer, a closed probe and a detecting probe for a mutation site; step 2, processing a sample and extracting DNA; step 3, applying the circulating tumor DNA EGFR detecting technology to the reagent kit, and establishing a PCR amplified reaction system. According to the circulating tumor DNA EGFR detecting technology disclosed by the invention, the specific MGB Blocker probe, the EGFR specific probe and the primer are adopted to detect the EGFR mutation in the circulating tumor DNA; the MGB Blocker probe is utilized to retard nonspecific amplification of the EGFR wild type genome and improves the sensitivity; the circulating tumor DNA EGFR detecting technology can be used for detecting the EGFR mutation in the circulating tumor DNA, is simple to operate, cheap in detection, capable of being popularized at a large scale and high in detection speed; the detecting process can be completed for about 2 hours.
Owner:张道允 +1
Digital PCR chip based on seaweed gel liquid drop and application thereof
ActiveCN106754245AImprove stabilityFusion does not occurBioreactor/fermenter combinationsBiological substance pretreatmentsPcr chipCirculating tumor DNA
The invention discloses a liquid drop capturing chip. The liquid drop capturing chip comprises a plurality of fluid micro channels arranged in parallel, wherein a plurality of liquid drop capturing structures are formed along the fluid micro channels at intervals; the liquid drop capturing structures comprise liquid drop entering ends and inhibition liquid drop flowing-out ends; and the inhibition liquid drop flowing-out ends are communicated with the adjacent fluid micro channels. The invention further discloses a fully integrated digital PCR chip based on a seaweed gel liquid drop and application thereof. The digital PCR chip based on the seaweed gel liquid drop, disclosed by the invention, can finish the full process from the generation of liquid drops to capture of the liquid drops and to the detection of the liquid drops. And moreover, seaweed gels are introduced; after the seaweed gels are solidified, the seaweed gel liquid drops are stable, cannot be fused and cannot affect the PCR amplification efficiency; and the method can be applied to the detection of circulating tumor DNA in the blood and other related biology and medical sciences; and due to the introduction of the seaweed gels, the application can be further expanded to single cell analysis and so on.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
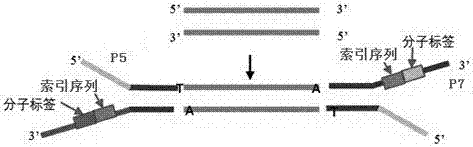
Method of detecting circular tumor DNA in free DNA of peripheral blood, kit and analytic method of sequencing result thereof
InactiveCN107475403AHigh sensitivityAvoid interferenceMicrobiological testing/measurementSequence analysisAbnormal tissue growthTail
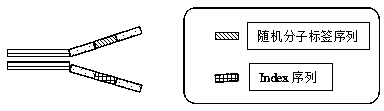
The embodiment of the invention discloses a method of detecting circular tumor DNA in free DNA of peripheral blood, a kit and an analytic method of a sequencing result thereof. The method of detecting circular tumor DNA comprises the following steps: S1, filling the tail end of cfDNA, and adding a basic group A into 3' of cfDNA; S2, connecting a joint to the tail end of the cfDNA treated in the S1 to obtain a connecting product, wherein the joint contains a molecular tag sequence and an Index sequence; S3, amplifying the connecting product with universal primers Primer 1.0 and Primer7; S4, capturing and enriching the amplified product obtained in the S3 by means of a probe; and S5, amplifying the enriched product obtained in the S4 by using the Primer 7 and Primer 5 and performing sequencing. The system error induced by PCR and sequencing can be removed effectively, so that the sequencing sensitivity of ctDNA is improved effectively.
Owner:深圳因和生物科技有限公司
Method and device for processing circulating tumor DNA repetitive sequence
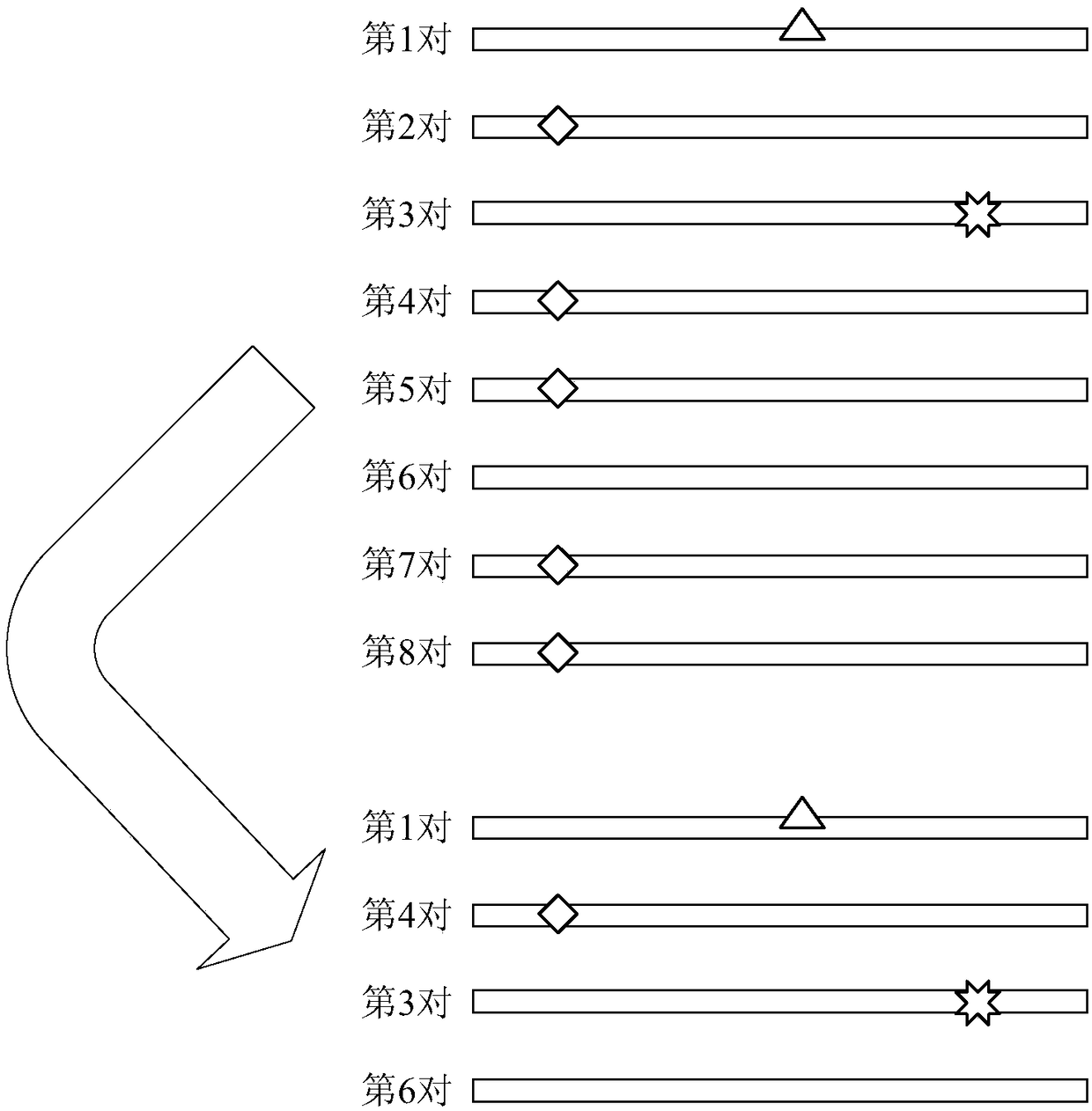
ActiveCN108229103AImprove processing accuracySequence analysisSpecial data processing applicationsReference genome sequenceCirculating tumor DNA
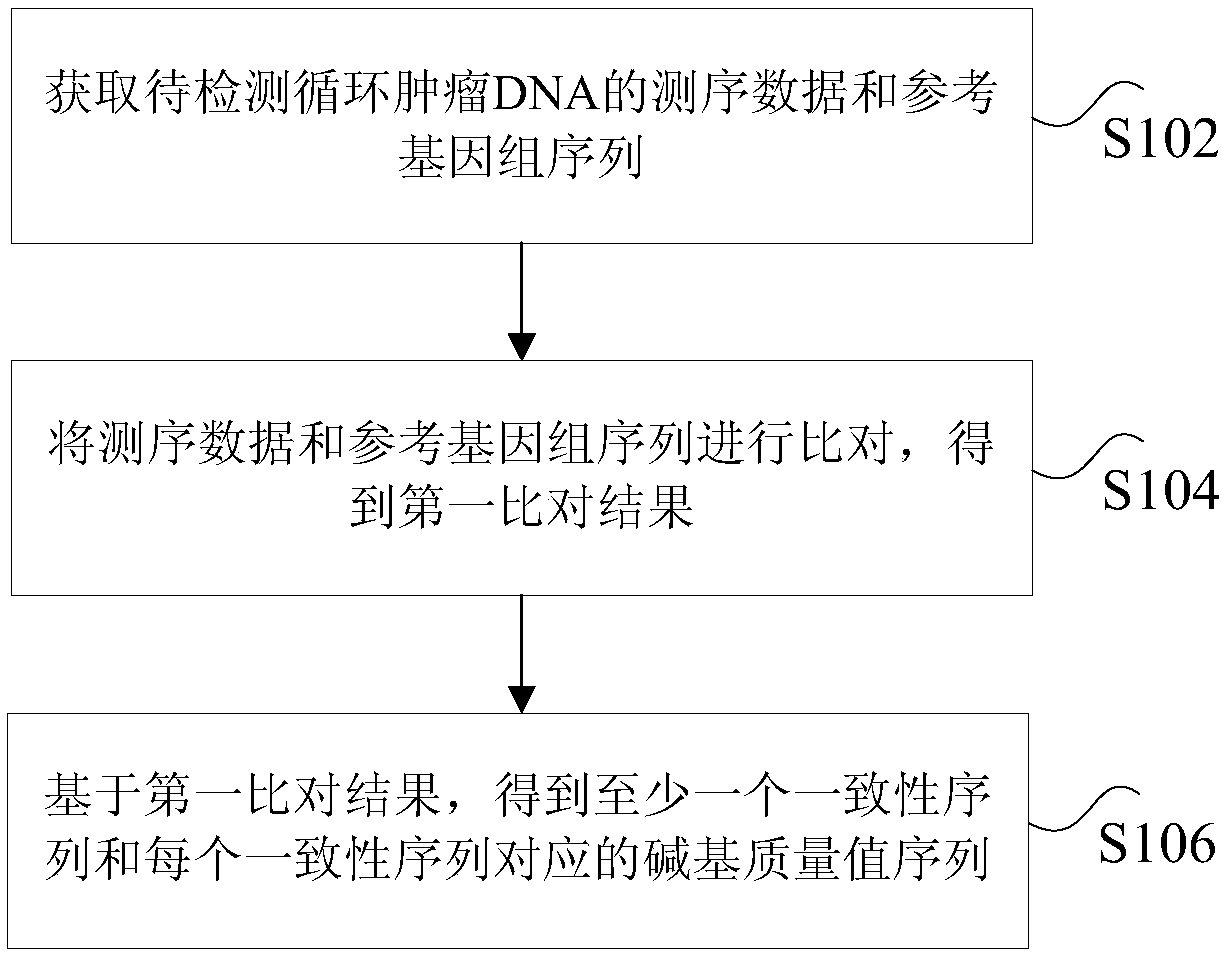
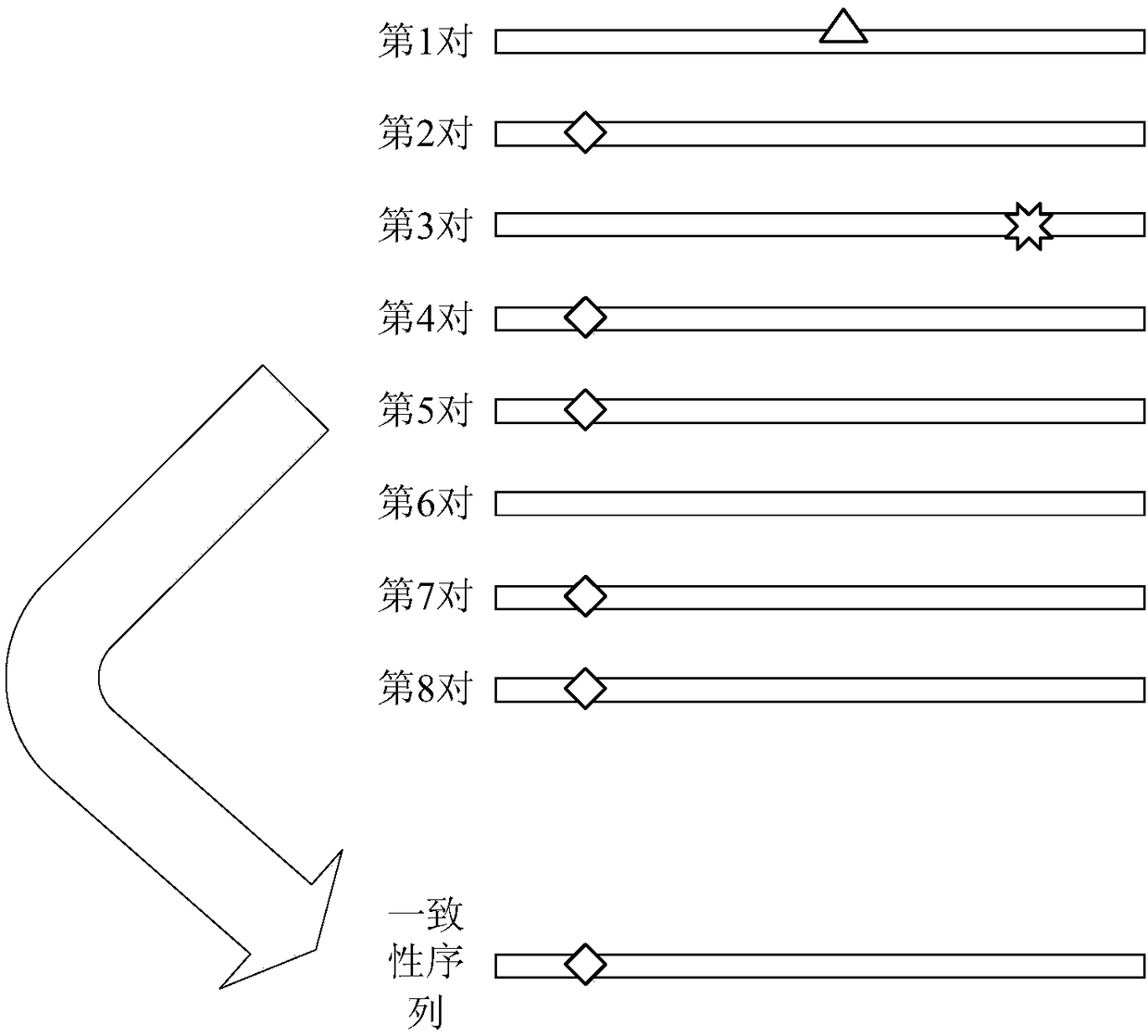
The invention discloses a method and device for processing a circulating tumor DNA repetitive sequence. The method comprises the steps that sequencing data and a reference genome sequence of to-be-detected circulating tumor DNA are obtained, wherein the sequencing data is obtained by carrying out high-throughput sequencing on the to-be-detected circulating tumor DNA and comprises a plurality of pairs of double-ended sequences; the sequencing data is compared with the reference genome sequence to obtain a first comparison result, wherein the first comparison result at least comprises genome positions, base sequences and corresponding base quality value sequences of the double-ended sequences; the type of each pair of double-ended sequences is determined on the basis of the first comparisonresult, wherein the types comprise independent sequences and repeated sequences. The method and the device solve the problem that a sequencing data processing method in the prior art is used for sequencing samples to delete or mark repetitive sequences and accordingly the accuracy is low.
Owner:GENE CRAB BIOTECH CO
Probe for detecting circulating tumor DNA (Deoxyribonucleic Acid) of human breast cancer and application of probe
InactiveCN105950739AStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationDiseaseIndividualized treatment
The invention discloses a probe for detecting a circulating tumor DNA (Deoxyribonucleic Acid) of a human breast cancer and application of the probe, belonging to the field of diagnosis and prevention of diseases. The invention designs a breast cancer selector which consists of DNA segments in mutation regions of related genes of the breast cancer. The circulating tumor DNA (ctDNA) of a breast cancer patient is subjected to liquid-phase hybridization capture by using the probe designed by the selector; after high-throughput sequencing, specific cancer gene variation of a patient is found, and a tumor load is monitored. Compared with a conventional detection means, the probe and the application thereof disclosed by the invention have the characteristics of no invasiveness, high throughout, good sensitivity and specificity, and the like; the probe and the application thereof can be used for better screening and early-stage diagnosis of the human breast cancer and are applied to individualized treatment of cancer.
Owner:HARBIN MEDICAL UNIVERSITY
Tumor burden as measured by cell free DNA
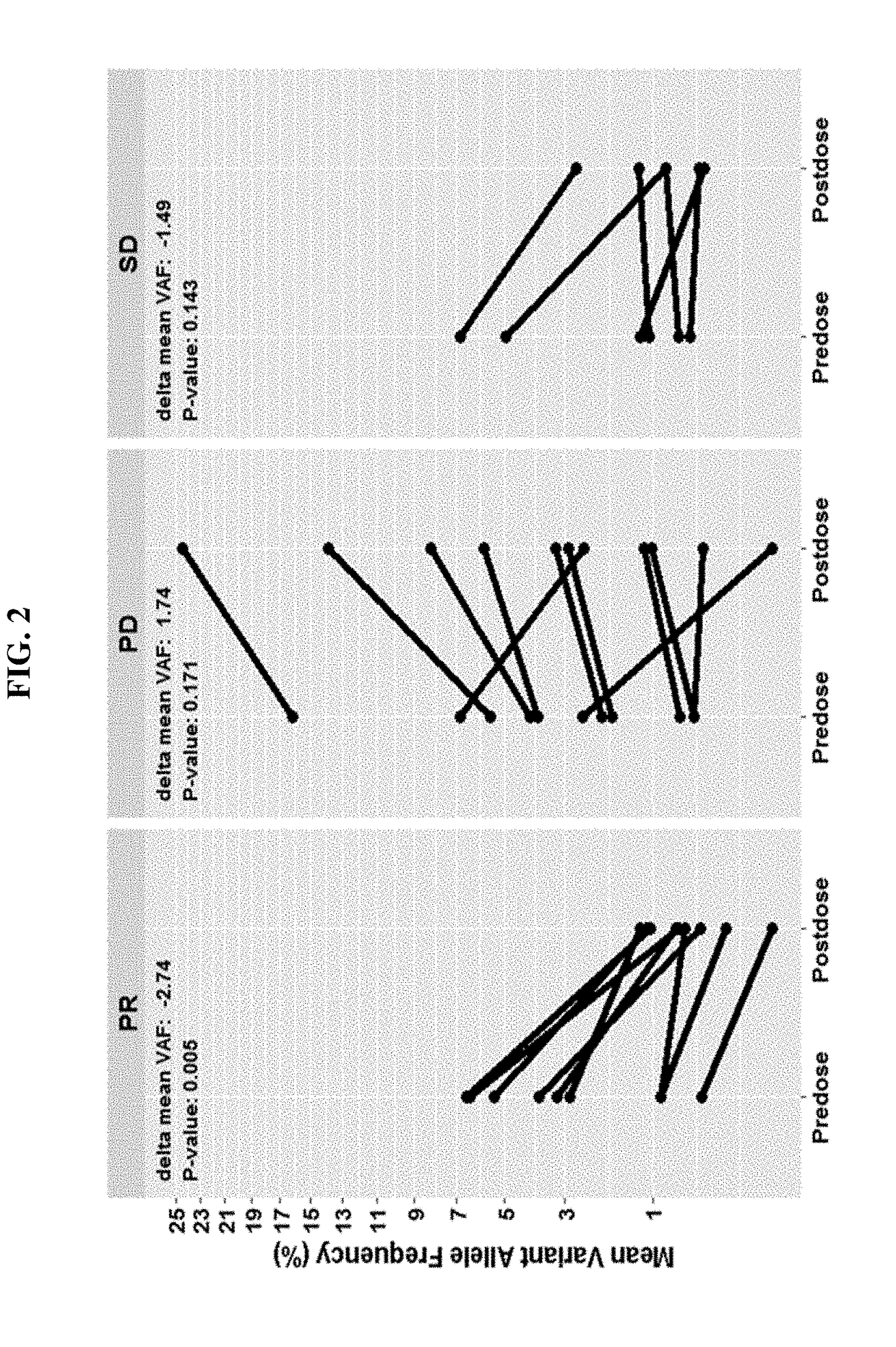
InactiveUS20180282417A1Good chanceLong survivalMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsCell freeAllele frequency
Disclosed are methods for treating cancer (e.g., solid tumor cancers, lung cancer, bladder head and neck cancer) with an anti-PD-L1 antibody in a patient identified as being responsive to anti-PD-L1 antibody therapy by detecting a mutation in one or more disclosed circulating tumor DNA (ctDNA) markers. Also disclosed are methods for determining the efficacy of anti-PD-L1 therapeutic antibody treatment in a patient having lung cancer or bladder cancer comprising detecting variant allele frequency in ctDNA in plasma samples and determining the difference of the variant allele frequency in ctDNA between the first and at least second plasma samples, wherein a decrease in the variant allele frequency in the at least second plasma sample relative to the first plasma sample identifies the anti-PD-L1 antibody treatment as effective. The disclosure also provides methods of identifying a subject having a cancer responsive to a therapy comprising an anti-PD-L1 antibody by detecting the expression of a mutation in one or more circulating tumor DNA (ctDNA) markers.
Owner:MEDIMMUNE LLC
Compositions and methods for detection of nucleic acid mutations
InactiveCN109415764ANucleotide librariesMicrobiological testing/measurementMultiplexCirculating tumor DNA
The invention provides methods and compositions for detecting a mutation in a target gene in a sample of blood or a fraction thereof, including in certain examples, a fraction that includes circulating tumor DNA. The methods can include a tiling PCR reaction, for example a one-sided multiplex tiling reaction. Virtually any type of mutation can be detected with the methods and compositions. In certain embodiments, gene fusions are detected. Improved PCR methods, especially for performing nested multiplex PCR reactions are provided.
Owner:NATERA
Ultra-sensitive detection of circulating tumor DNA through genome-wide integration
PendingCN112601826AMedical simulationMicrobiological testing/measurementDiseaseCirculating tumor DNA
Owner:CORNELL UNIVERSITY +1
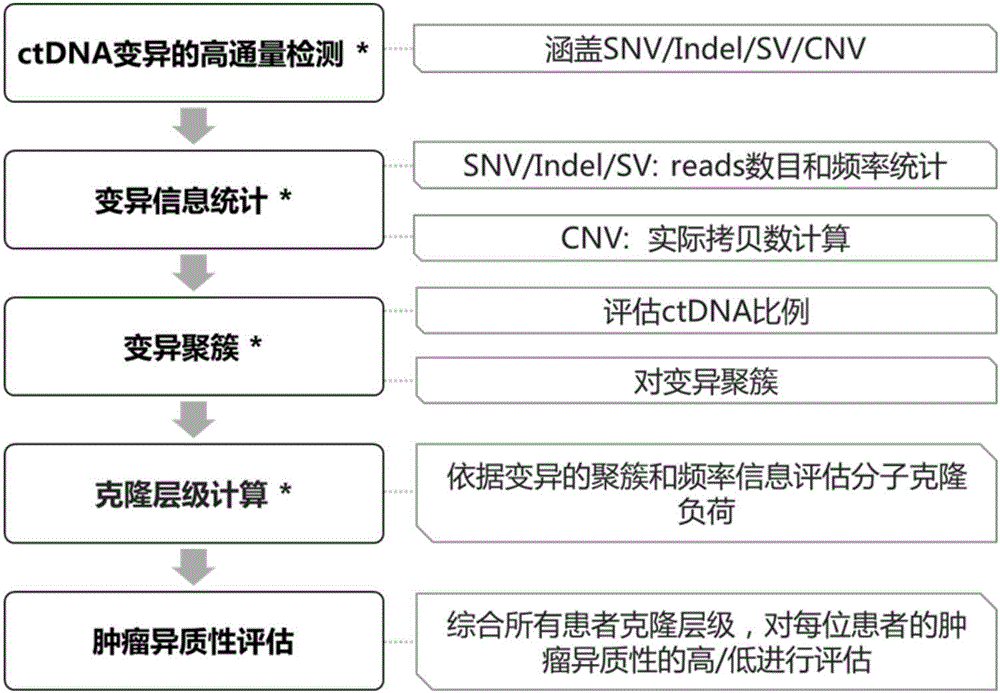
System and method for tumor heterogeneity assessment
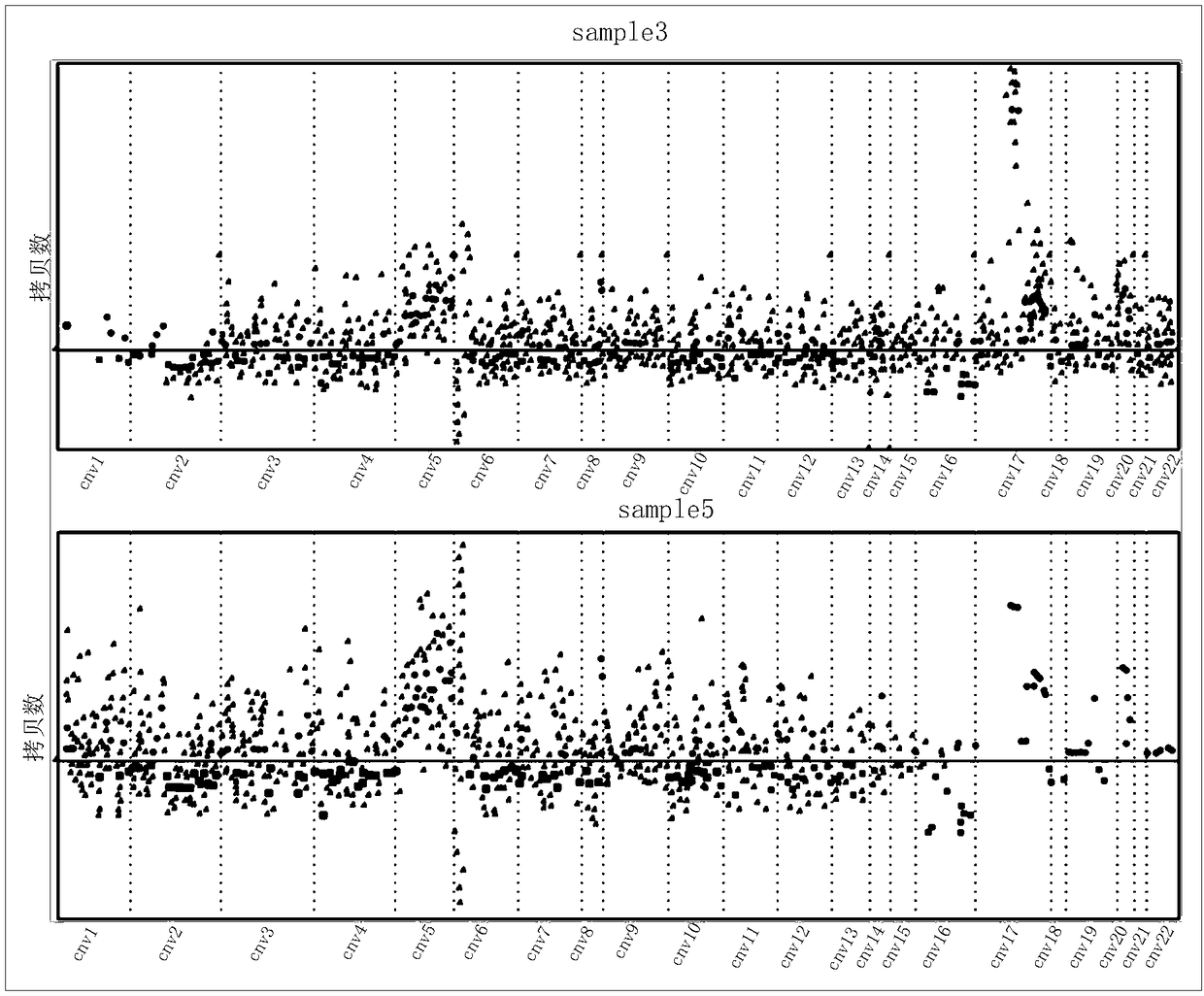
ActiveCN106676178ARich in featuresAccurate and Reasonable AssessmentMicrobiological testing/measurementProteomicsCirculating tumor DNATumor heterogeneity
The invention discloses a system and a method for tumor heterogeneity assessment and particularly provides a molecular clone analysis method. On the basis of various types of variation detection results of high-throughput sequencing in circulating tumor DNA, all variations are divided into different molecular clones to realize tumor heterogeneity assessment by molecular clone levels. By adoption of the method, tumor heterogeneity assessment based on ctDNA high-throughput variation detection is realized to effectively assist in making of tumor prognosis and treatment schemes.
Owner:BEIJING GENEPLUS TECH +1
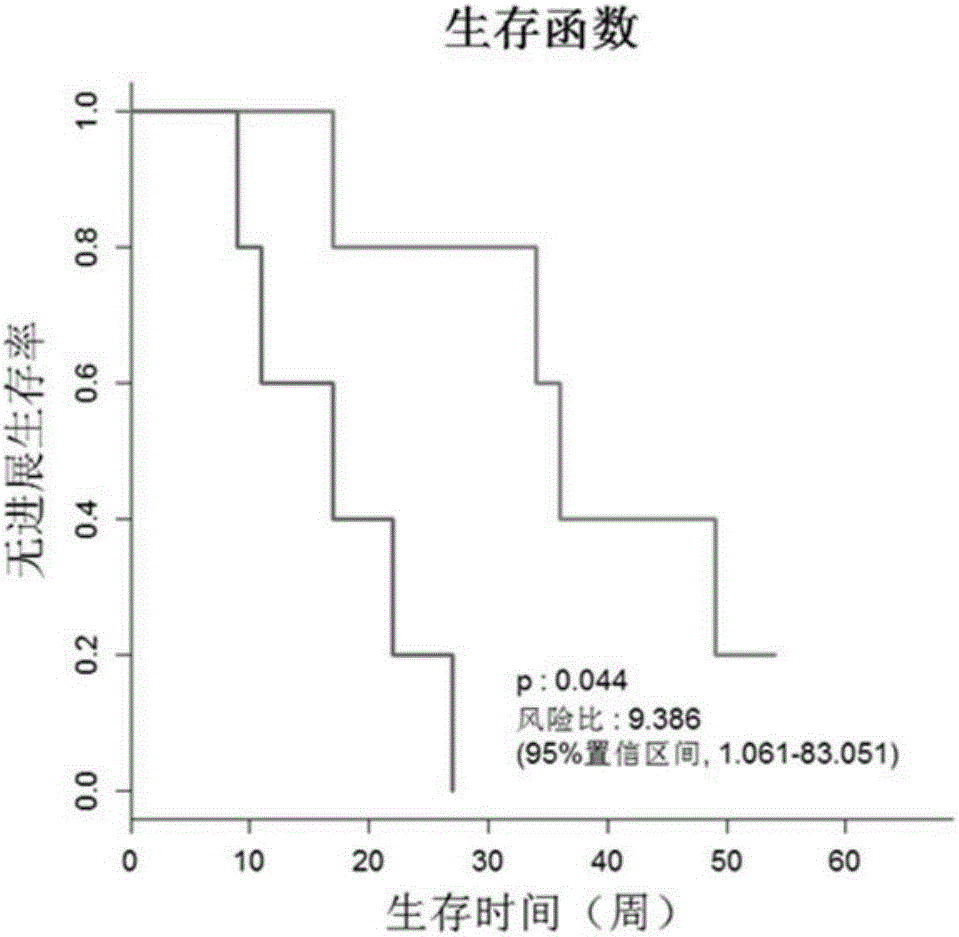
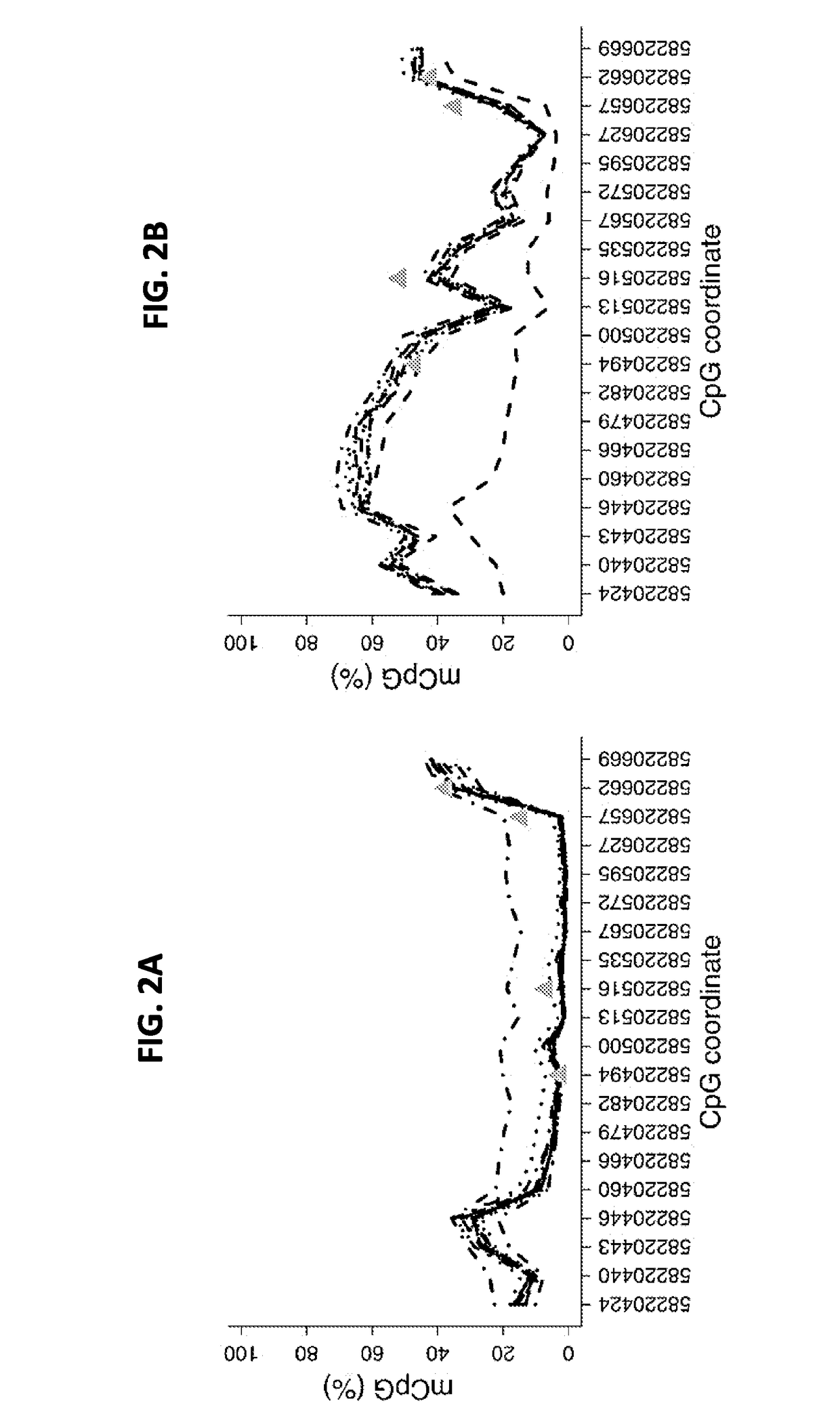
Detection kit for TMEM101 gene methylation in human peripheral blood circulating tumor DNA for early screening of endometrial cancer
ActiveCN113215264AImprove complianceSimple and fast operationMicrobiological testing/measurementBiostatisticsOncologyGene targeting
The invention discloses a detection kit for TMEM101 gene methylation in human peripheral blood circulating tumor DNA for early screening of endometrial cancer. The detection kit is applied to early screening of endometrial cancer and comprises a methylation specific primer of a TMEM101 gene target site, a hydrolysis probe which is specific to the TMEM101 gene, and a primer of a reference gene and a hydrolysis probe of the reference gene, wherein the target site of the TMEM101 gene is at least one CpG site in an upstream and downstream 2000bp interval of a transcription start site of the TMEM101 gene; the reference gene is one or more of GAPDH and beta-actin; and the kit comprises a PCR pre-amplification reaction system and a PCR amplification reaction system. According to the provided detection kit for TMEM101 gene methylation in human peripheral blood circulating tumor DNA for early screening of endometrial cancer, the technical defects that in the prior art, the process is tedious, the detection time is long, the false positive is high, and the early screening of the endometrial cancer blood cannot be traced are overcome.
Owner:上海伯豪生物技术有限公司
Methods for cancer detection and monitoring by means of personalized detection of circulating tumor DNA
The invention provides methods for detecting single nucleotide variants in breast cancer, bladder cancer, or colorectal cancer. Additional methods and compositions, such as reaction mixtures and solidsupports comprising clonal populations of nucleic acids, are provided. For example, provided here is a method for monitoring and detection of early relapse or metastasis of breast cancer, bladder cancer, or colorectal cancer, comprising generating a set of amplicons by performing a multiplex amplification reaction on nucleic acids isolated from a sample of blood or urine or a fraction thereof from a patient who has been treated for a breast cancer, bladder cancer, or colorectal cancer, wherein each amplicon of the set of amplicons spans at least one single nucleotide variant locus of a set ofpatient-specific single nucleotide variant loci associated with the breast cancer, bladder cancer, or colorectal cancer; and determining the sequence of at least a segment of each amplicon of the setof amplicons that comprises a patient-specific single nucleotide variant locus, wherein detection of one or more patient-specific single nucleotide variants is indicative of early relapse or metastasis of breast cancer, bladder cancer, or colorectal cancer.
Owner:NATERA
Cancer detection methods
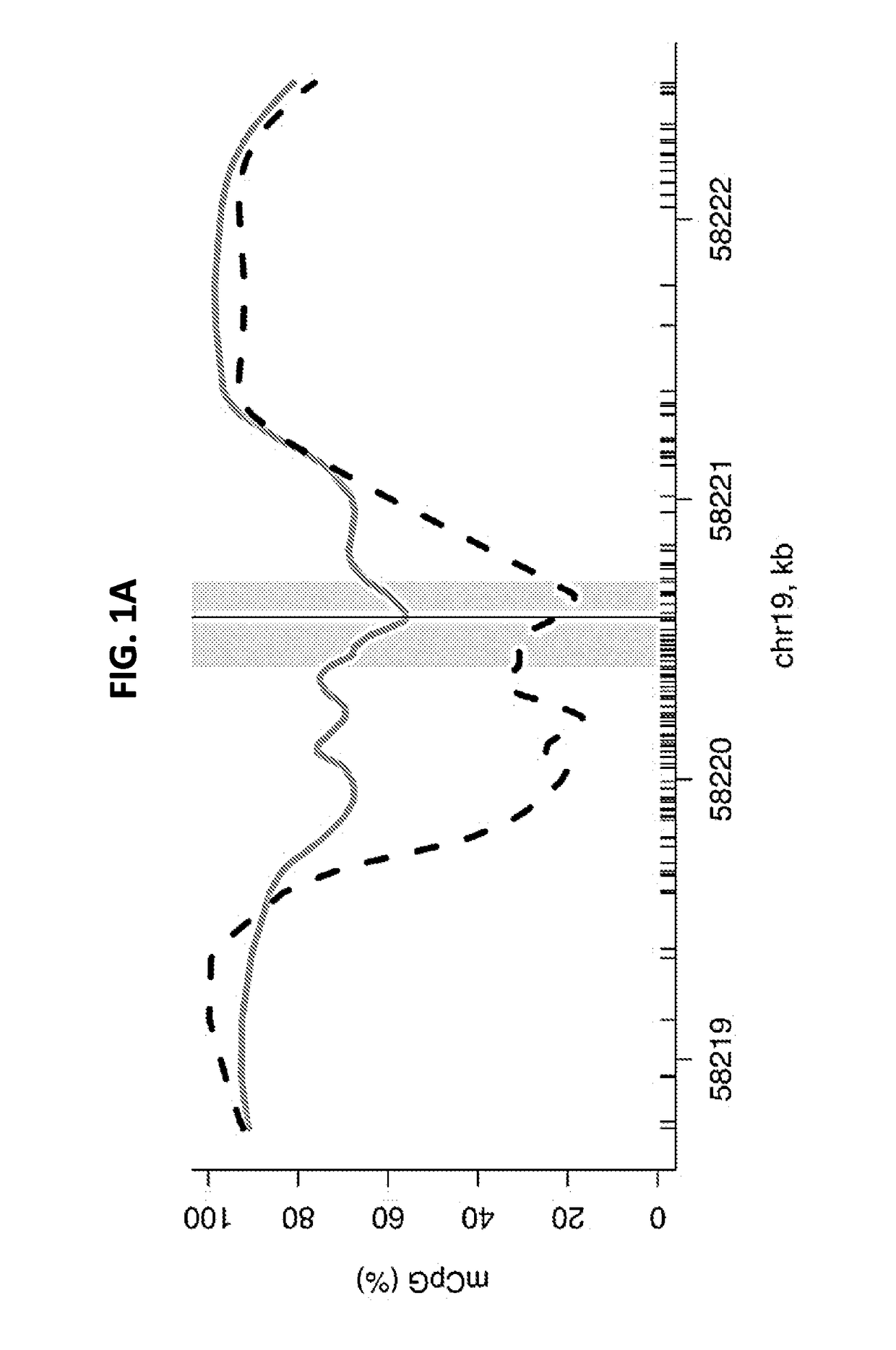
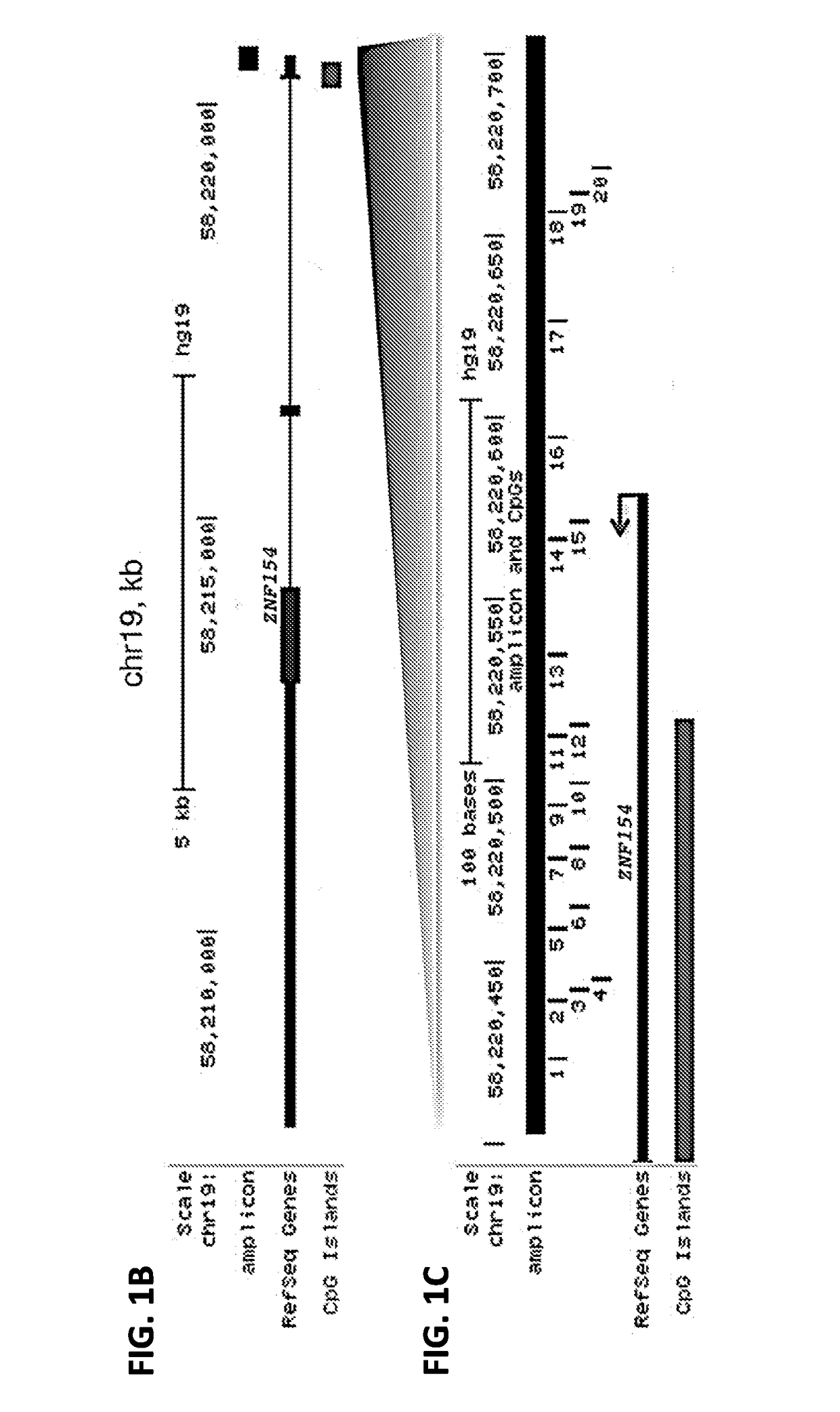
ActiveUS20180216195A1Elevated methylation levelMicrobiological testing/measurementCirculating tumor DNACancer detection
The present application provides methods for the detection and diagnosis of cancer. In one aspect, the application provides methods for detecting the presence of cancer in an individual by detecting the methylation state of a region in the promoter of the ZNF154 gene. Methods are provided for detection and diagnosis of cancer from circulating tumor DNA which are minimally invasive and have diagnostic utility across different types and sub-types of cancer. In a further aspect, bioinformatics methods are provided to analyze the methylation state of the ZNF154 promoter and relate the methylation state to the likelihood of cancer in the individual.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
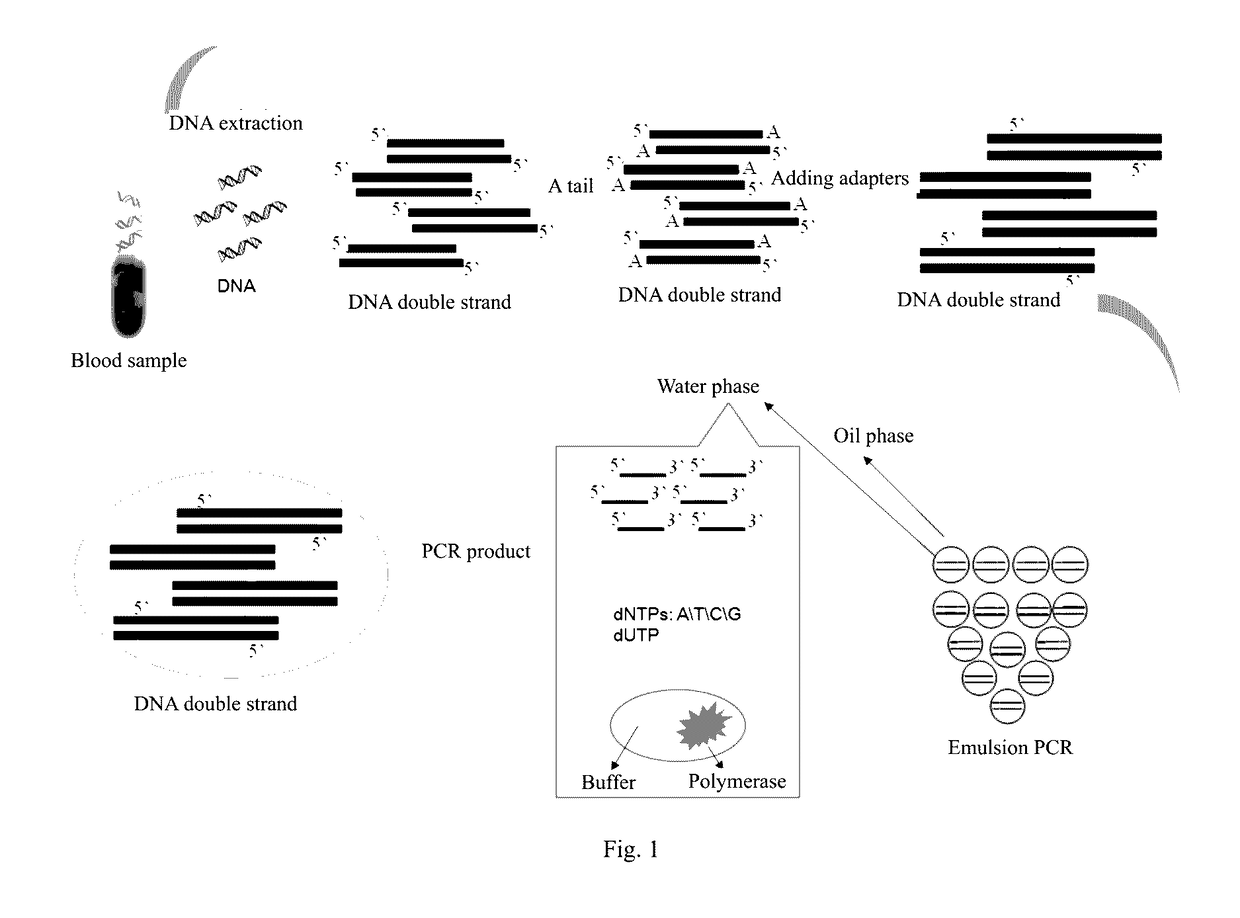
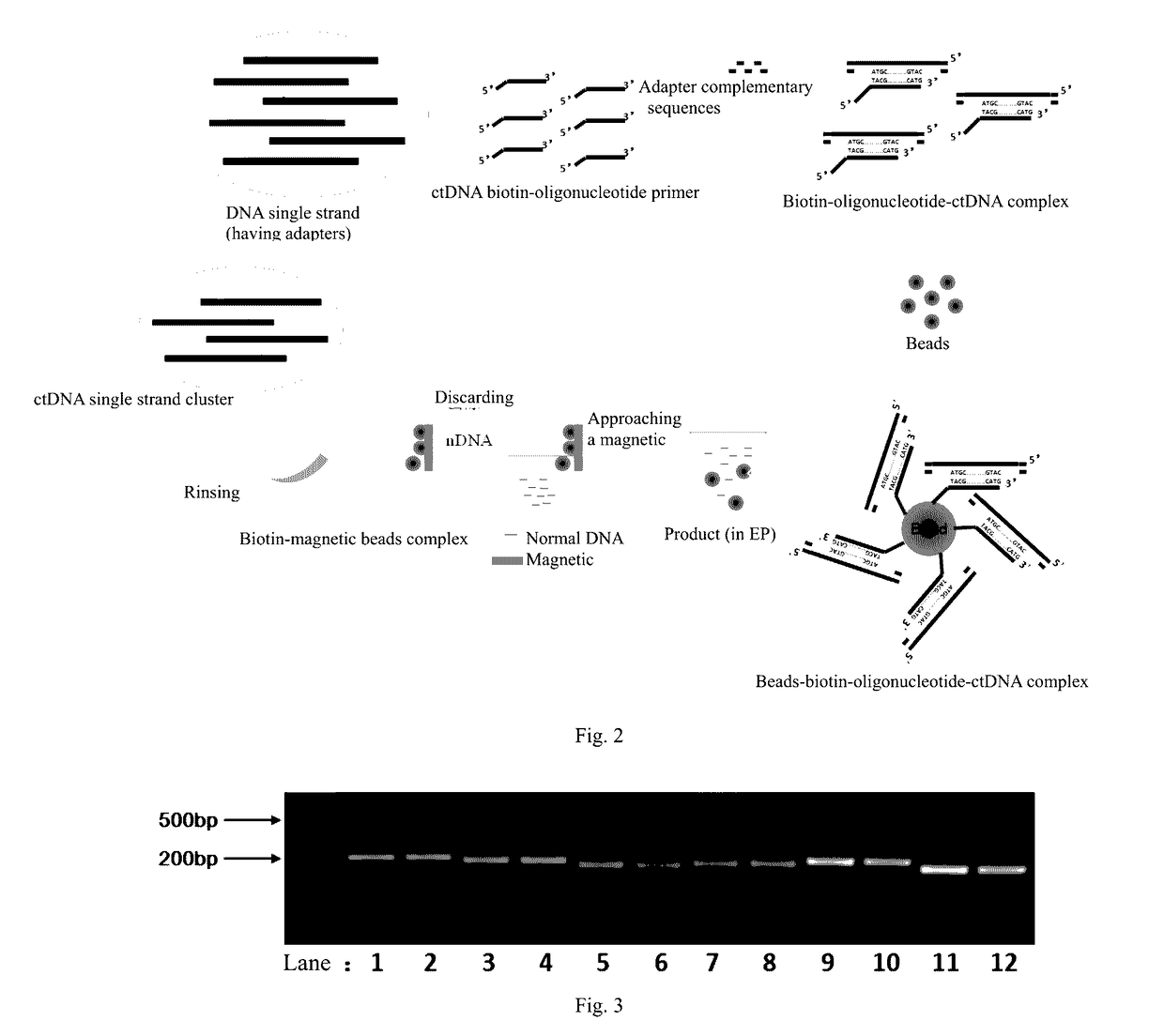
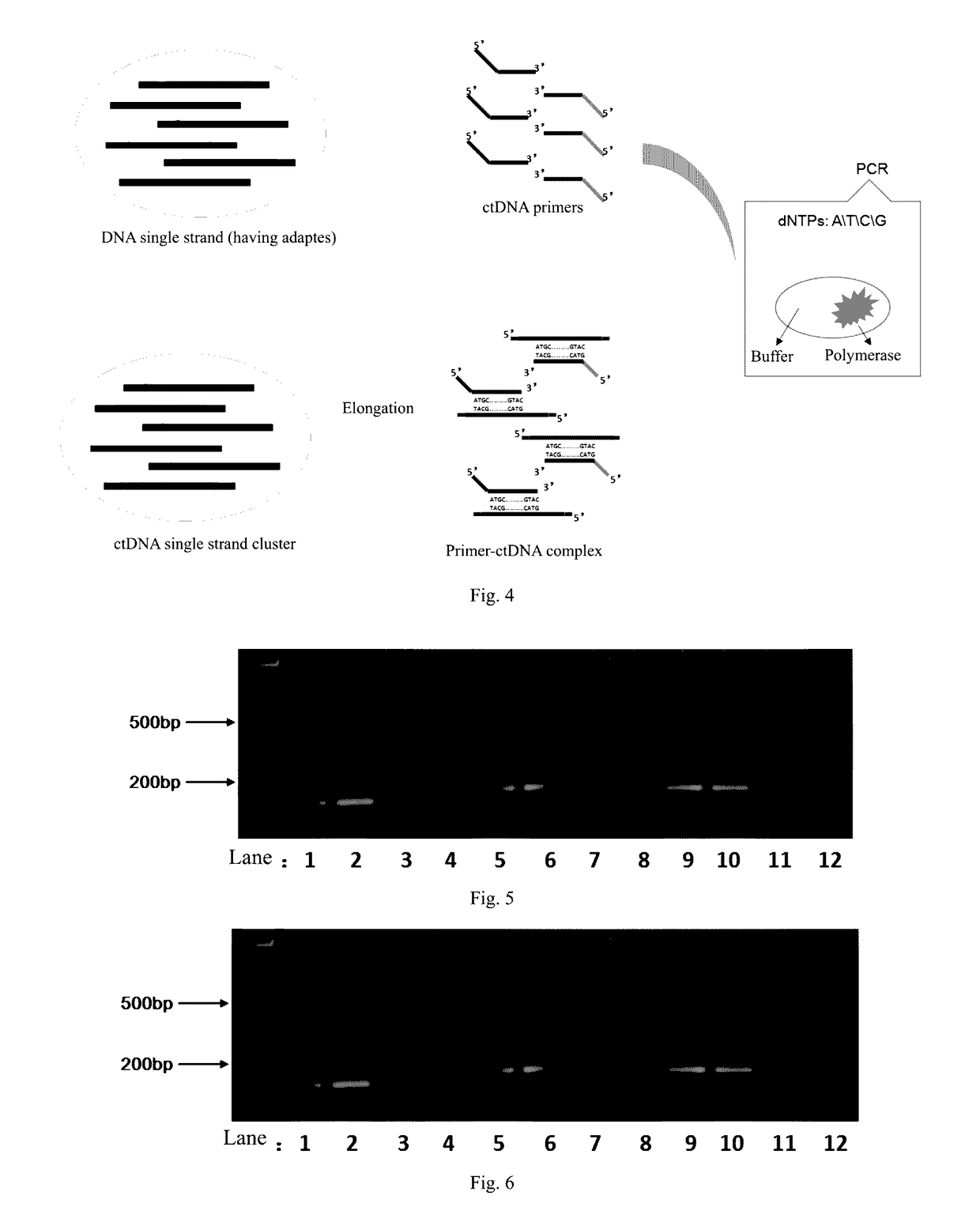
Method and reagent for enrichment of circulating tumor DNA
ActiveUS20170067117A1Efficient captureSufficient amountMicrobiological testing/measurementForward primerPolymerase L
The present invention provides a method and a reagent for enrichment of circulating tumor DNA, the method comprising the steps of mixing a water phase and an oil phase and shaking the mixture to prepare an emulsion PCR reaction system, and performing emulsion PCR amplification, wherein the water phase comprises peripheral blood plasma DNA as template DNA, a forward primer and a reverse primer, dNTPs, a PCR buffer and a DNA polymerase, the peripheral blood plasma DNA having adapter sequences connected to both ends thereof, and the forward primer and the reverse primer being complementary to the adapter sequences at the two ends respectively; separating the water phase from the oil phase following the emulsion PCR amplification to obtain a PCR amplification product in the water phase; and capturing circulating tumor DNA in the PCR amplification product in the water phase by using a probe sequence that specifically binds to the circulating tumor DNA. The method of the present invention is capable of performing single-molecule high-fidelity ultramicro parallel amplification and effectively capturing peripheral blood plasma ctDNA to provide adequate amount of ctDNA to be used for subsequent sequencing detection.
Owner:SHENZHEN HAPLOX BIOTECH
Kit for amplifying Her2 (Human epidermal growth factor receptor-2) gene in human peripheral blood circulating tumor DNA (Deoxyribonucleic Acid)
InactiveCN106011239AIncreased sensitivityImprove reliabilityMicrobiological testing/measurementFluorescenceCirculating tumor DNA
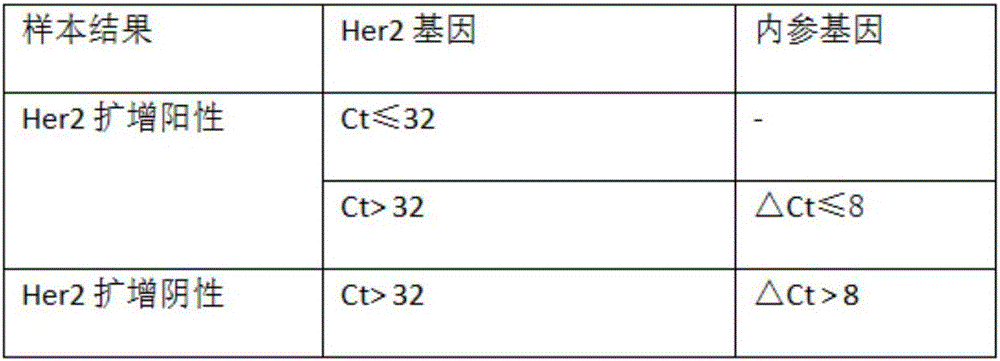
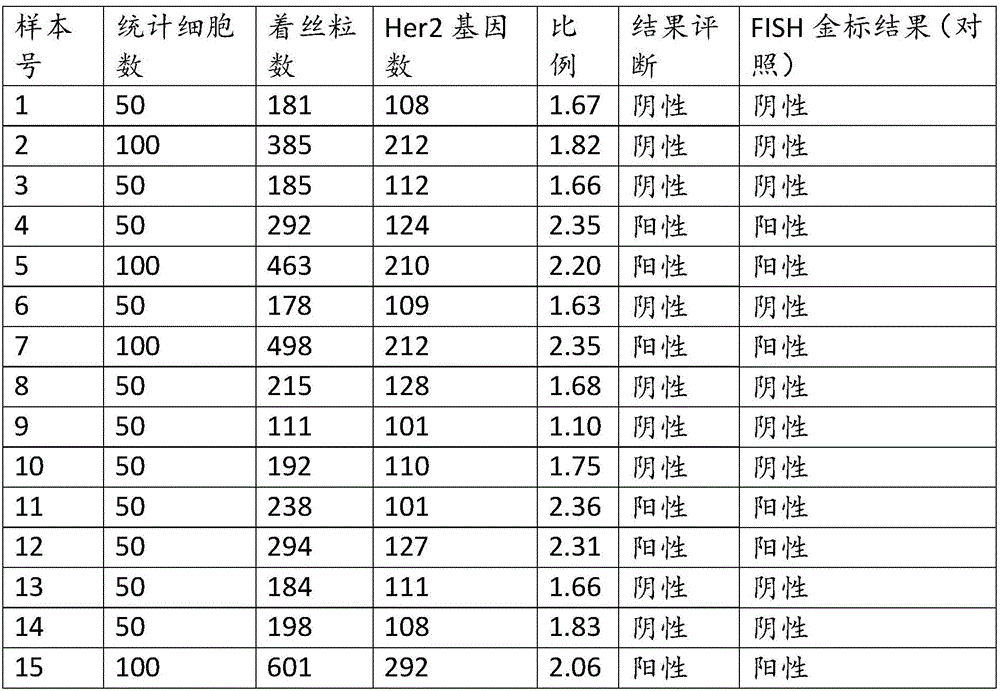
The invention discloses a kit for amplifying a Her2 (Human epidermal growth factor receptor-2) gene in human peripheral blood circulating tumor DNA (Deoxyribonucleic Acid). By adopting component setting of the kit and a result reading scheme, a detection process is simple and convenient and the operability is strong. A detection result is reliable and has good specificity; meanwhile, a real-time fluorescence PCR (Polymerase Chain Reaction) technology is used for detecting an amplification level of the Her2 gene in a breast cancer tissue specimen through calculating a deltaCt value; the kit can be used for a plurality of research fields including breast cancer auxiliary diagnosis carried out through detection of ctDNA of the blood, clinical application, prognosis and the like.
Owner:BEIJING ABACE BIOTECH
Method and detection kit to judge whether solid tumors are suited for immunotherapy
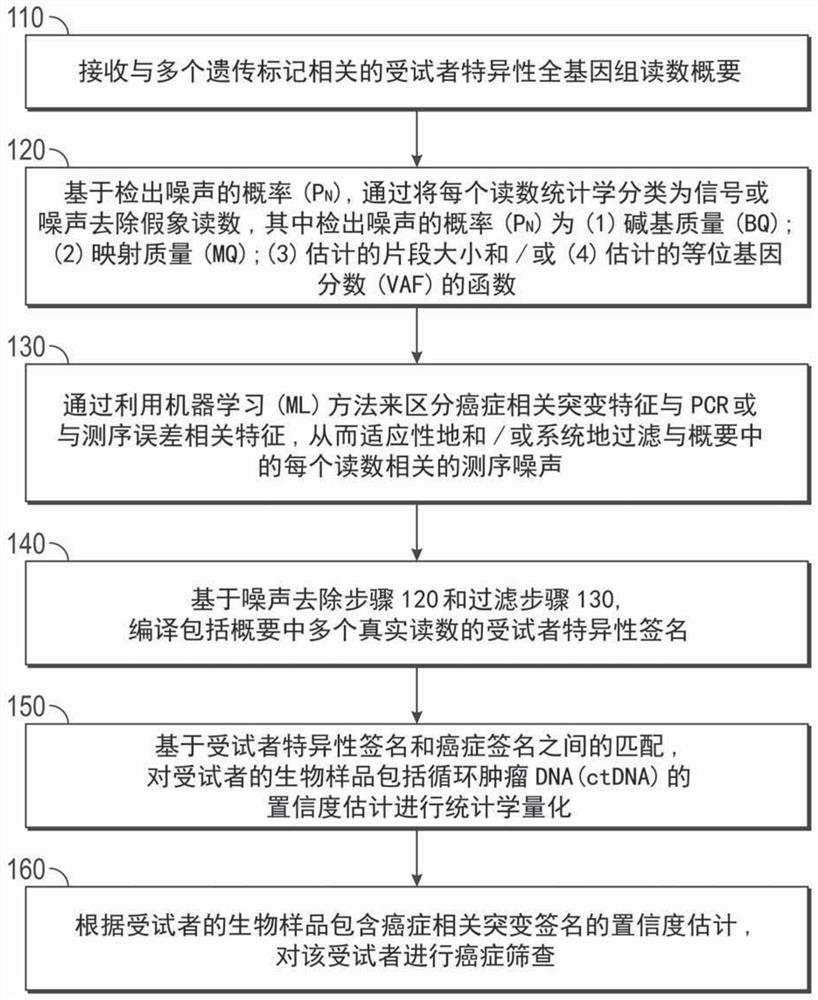
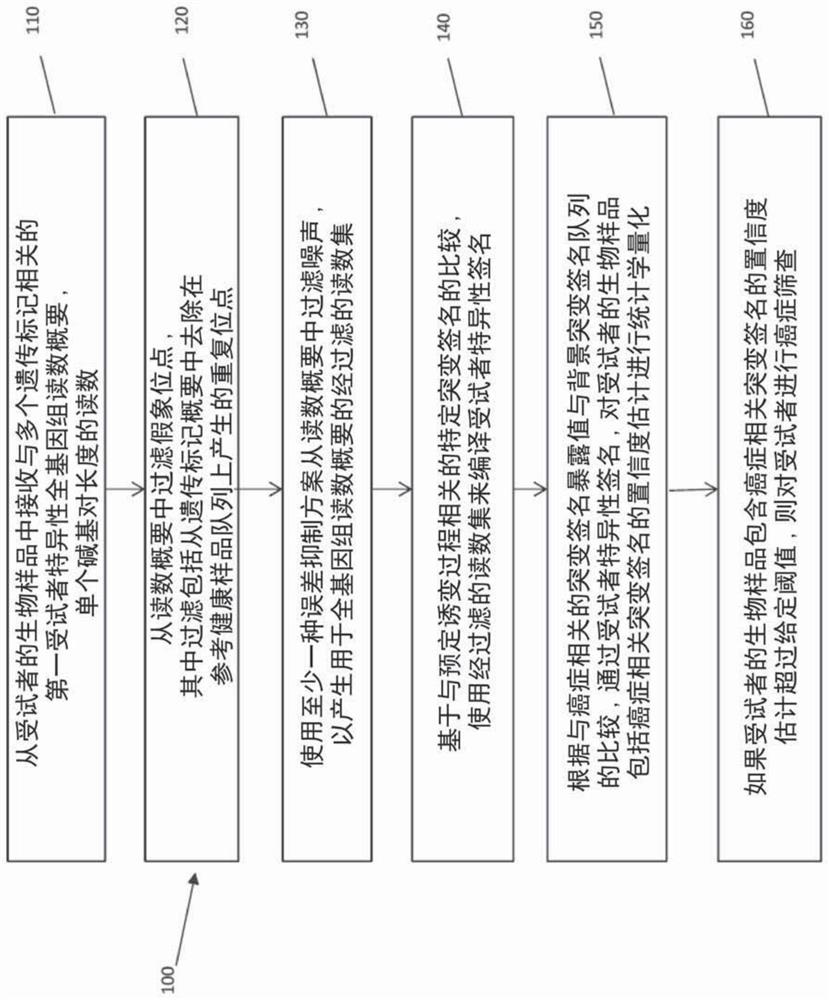
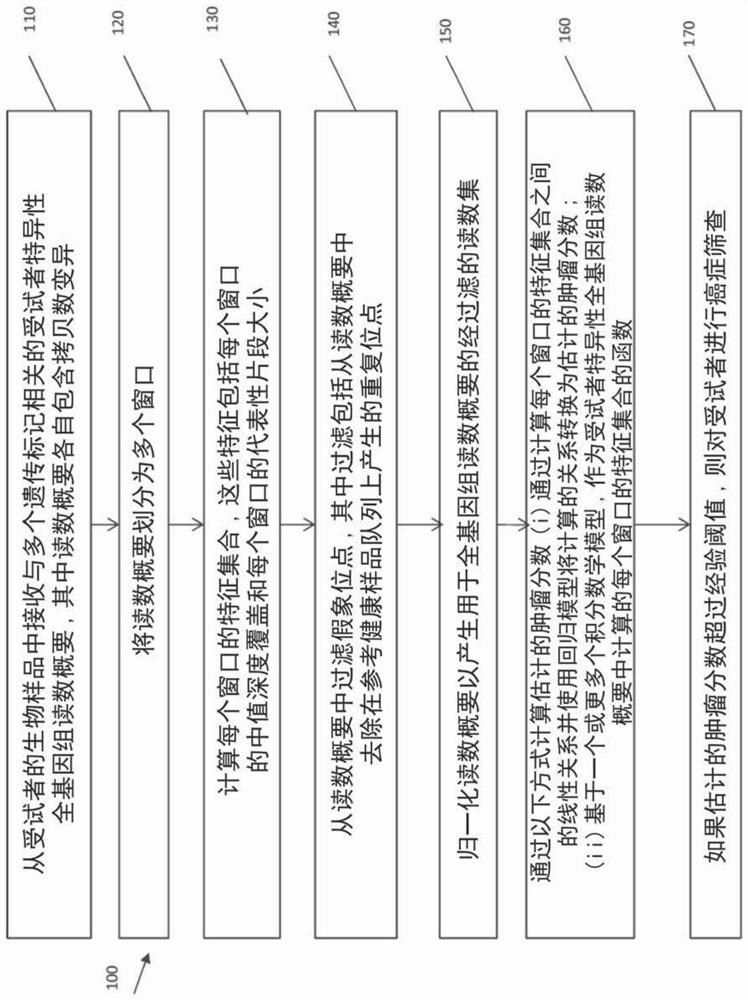
ActiveCN108624650AIncreased objective response rateMicrobiological testing/measurementMaterial analysisPeripheral blood mononuclear cellCD16
The invention provides a detection kit to judge whether solid tumors are suited for immunotherapy. The detection kit includes detection reagents for detecting the indexes: tumor mutation load of peripheral blood circulating tumor DNA, HLA typing, HLA state of loss of heterozygosity, PD-L1 membrane expression positive tumor cell percentage, infiltration level of CD8+ immune cells, infiltration level of FOXP3+ immune cells, mRNA expression score of T-cell inflammation related genes, percentage of CD14+CD16-HLA-DR+ monocytes in peripheral blood mononuclear cells, and micro-satellite instability.The invention also provides, correspondingly, a method to judge whether solid tumors are suited for immunotherapy. The kit and method herein can be used to screen patients with solid tumors so as to provide significantly increased objective response rate and have a promising application prospect in terms of clinical screening of patients with solid tumors suited for immunotherapy.
Owner:LEPU MEDICAL TECH (BEIJING) CO LTD
Method and kit for enriching circulating tumor DNA on basis of multiplex PCR
ActiveCN110117574AReduce lossesHigh detection sensitivityMicrobiological testing/measurementTumor/cancer cellsGene targetsCirculating tumor DNA
The invention discloses a method and kit for enriching circulating tumor DNA on the basis of multiplex PCR. The method comprises the steps of (1), designing multiple PCR primer pairs, wherein PCR primers comprise an amplifying primer sequence used for amplifying a gene target region and a primer ligation sequence, and the primer ligation sequence is used for being linked with a universal sequencing linker of a sequencing platform; (2), taking a template containing the circulating tumor DNA and making the template and the PCR primer pairs in step (1) subjected to a first PCR reaction to obtaina first PCR reaction product; (3), adding a combined enzyme into the first PCR reaction product to digest residual PCR primers; (4), adding the universal sequencing linker into the first PCR reactionproduct and carrying out a second PCR reaction to obtain a sequencing library. The method and the kit have the effect of reducing sample loss.
Owner:常州桐树生物科技有限公司
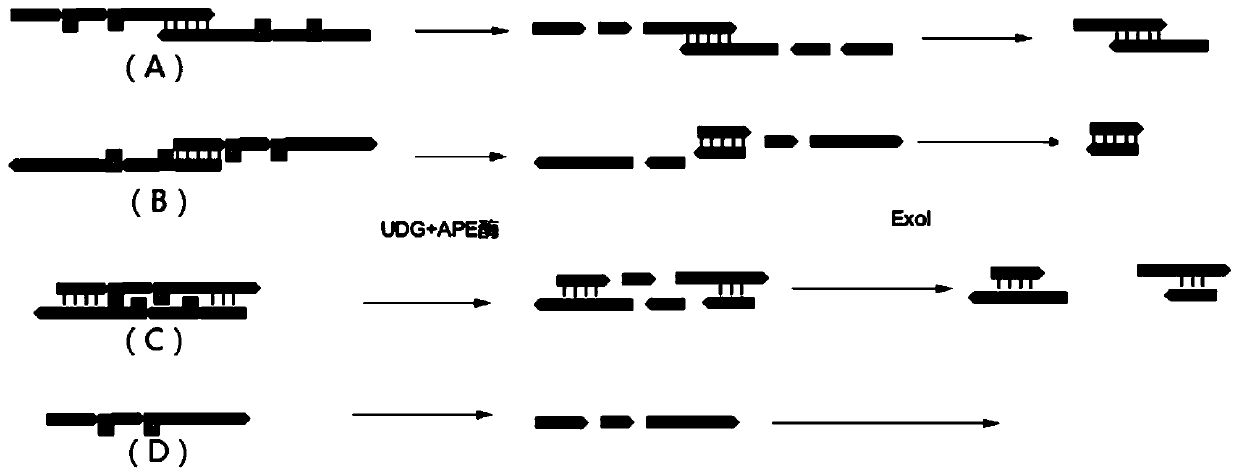
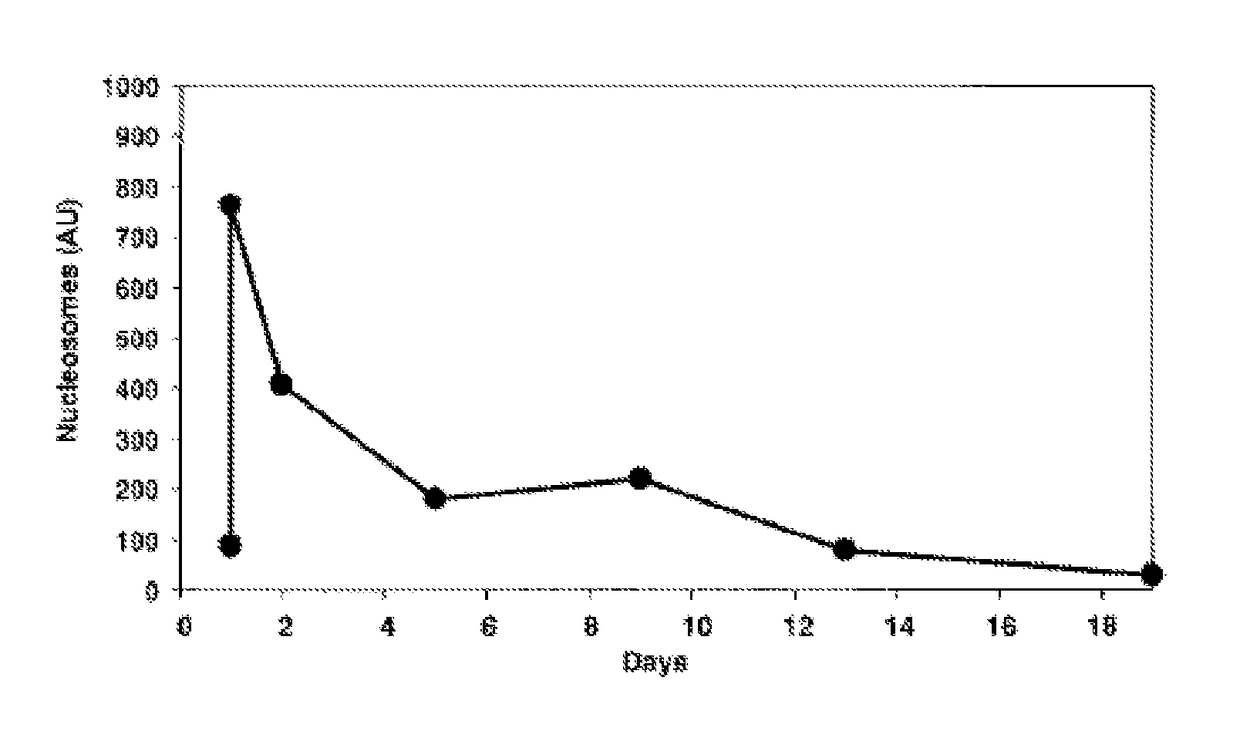
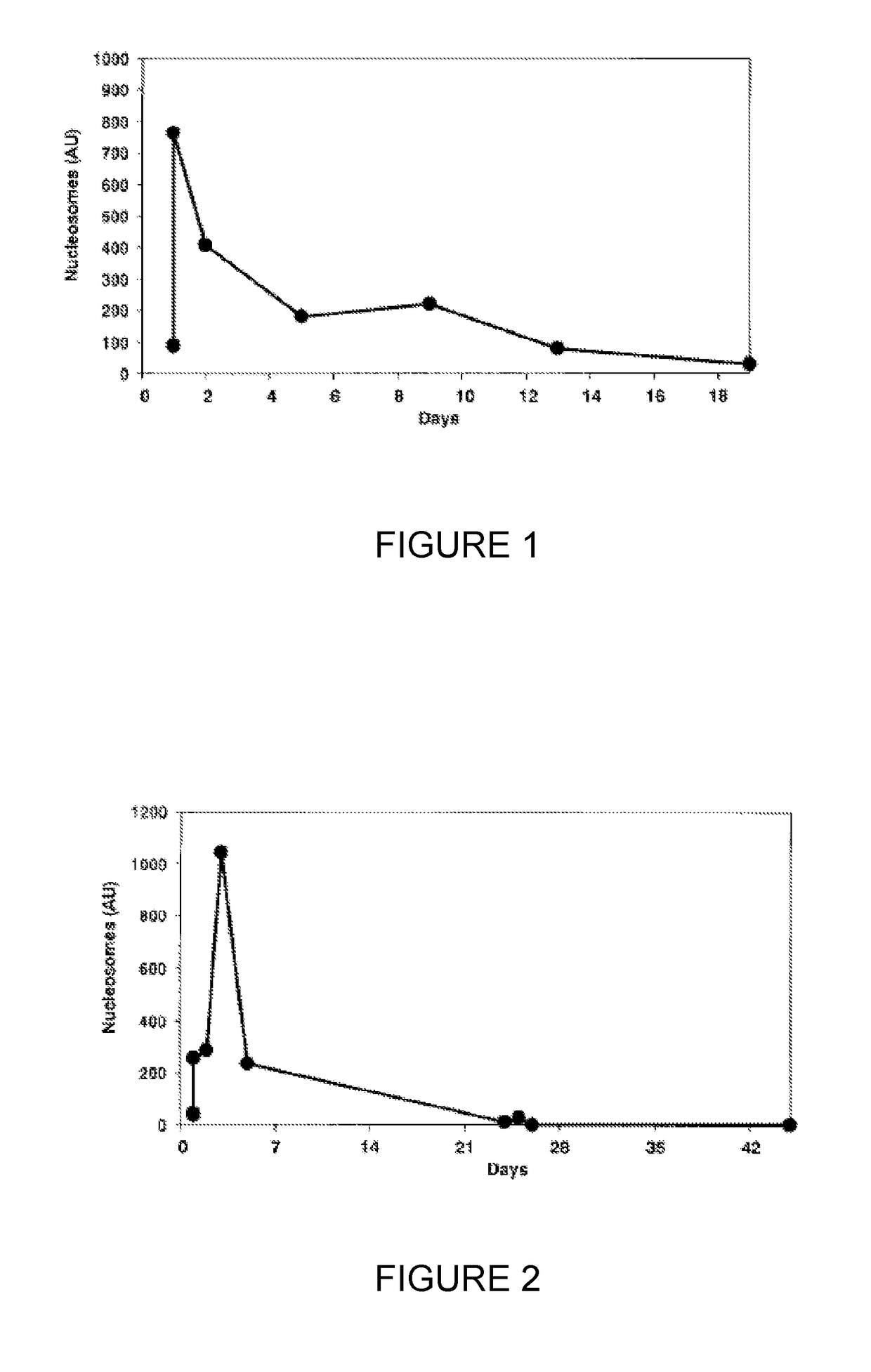
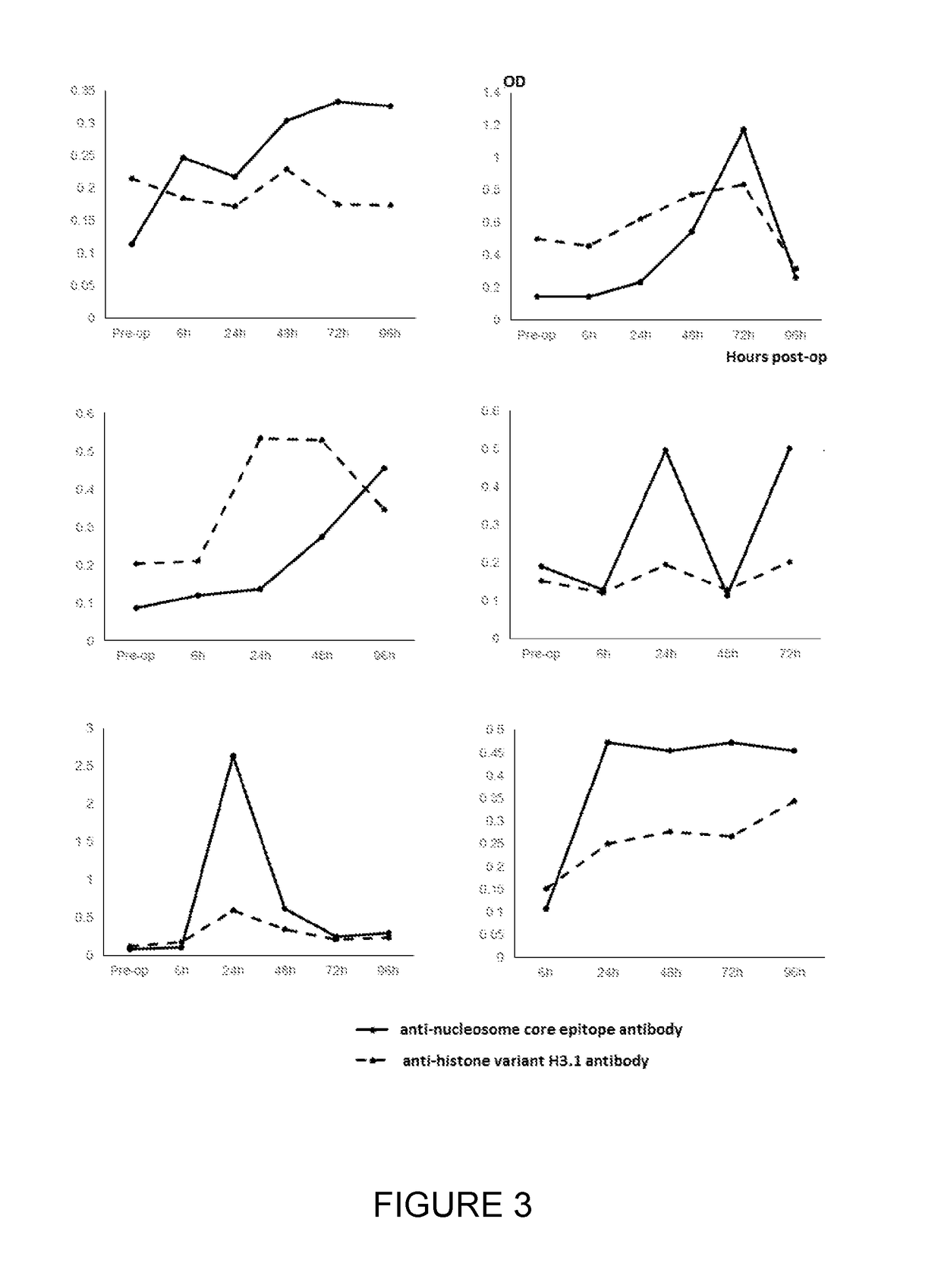
Method for the enrichment of circulating tumor DNA
InactiveUS20180024141A1Mass spectrometric analysisMaterial analysis by electric/magnetic meansCell freeCirculating tumor DNA
The invention relates to the use of histone binding agents for detecting, isolating and / or purifying cell free nucleosomes of tumor originor circulating tumor DNA from a biological sample. The invention also relates to methods and kits using said histone binding agents.
Owner:BELGIAN VOLITION SPRL
Primer set and reagent kit for detecting mutation of lung cancer related genes in human circulating tumor DNA and use method of reagent kit
ActiveCN110144399AHigh sensitivityIncrease signal valueMicrobiological testing/measurementDNA/RNA fragmentationBiotin-streptavidin complexMagnetic bead
The invention discloses a primer set and reagent kit for detecting mutation of lung cancer related genes in human circulating tumor DNA and a use method of the reagent kit. 49 gene variation sites forassessing specificity and sensibility of risk that human suffer from lung cancer, of 5 genes are provided, and the sites are subjected to gene analysis and detection through a MassARRAY nucleic acidmass spectrum technique. When the reagent kit is in a single-basic-group extension, only ddNTPs for extending mutant genes is added, the ddNTPs has biotin marks, magnetic beads having streptavidin marks are used for capturing extension products, and signal interference of impurity peaks is removed, so that the signal value of mutation genes in the extension products is greatly increased, and the sensitivity of the reagent kit is increased. When the method is used for detecting circulating tumor DNA samples, the sensitivity under 20ng of sampling quantity can be as low as 0.1%. Compared with qPCR and a bibasic sequencing platform, the method has the advantages of being flexible in design, high in accuracy, low in cost, simple and convenient to operate and the like.
Owner:中源维康(天津)医学检验所有限公司
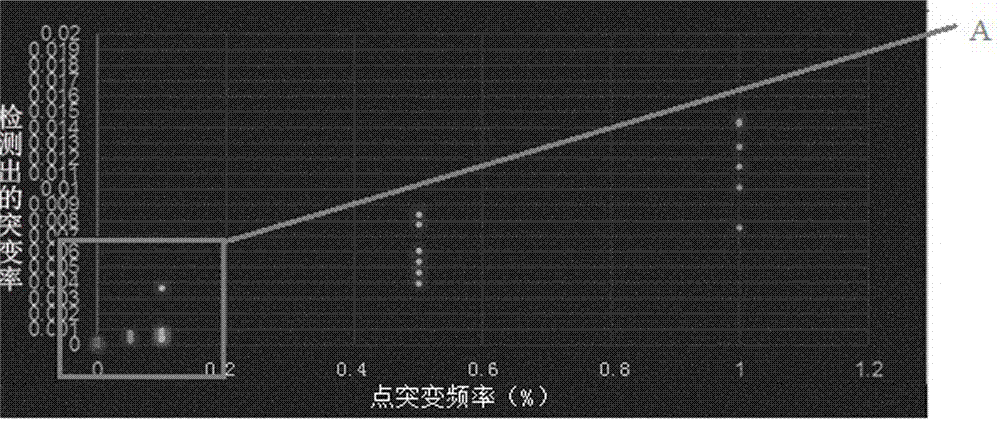
Detection method for low-frequency mutation of circulating tumor DNA
ActiveCN108866174AReduce repetition rateEliminate errorsMicrobiological testing/measurementCirculating tumor DNATrue positive rate
The invention discloses a detection method for low-frequency mutation of circulating tumor DNA (which is called as ctDNA for short in following). The method comprises the following steps: S1, extracting cfDNA from blood plasma; S2, repairing the tail end of the extracted cfDNA and adding A at the 3' end; S3, performing connector connection on a tail end repaired product, wherein a connector contains a random molecular tag sequence and contains an index sequence for distinguishing different samples; S4, performing PCR amplification on a connector connection product; S5, obtaining a target library through a probe; S6, sequencing through illumina Miseq, illumina Nextseq, illumina Hiseq platforms and analyzing offline data. The method is suitable for probe target capturing sequencing, can reduce an offline data repetition rate, can remove PCR and errors generated in a sequencing process, can improve ctDNA detection flexibility and specificity and can reduce a false positive rate.
Owner:厦门基源医疗科技有限公司
Method and device for processing repetitive sequences of circulating tumor deoxyribonucleic acid (DNA)
ActiveCN108595918AImplement deduplicationReduce human errorSequence analysisSpecial data processing applicationsRepetitive SequencesReference genome sequence
The invention discloses a method and device for processing repetitive sequences of circulating tumor deoxyribonucleic acid (DNA). The method comprises the following steps of: obtaining sequencing dataof to-be-detected circulating tumor DNA and a reference genomic sequence, wherein the sequencing data are obtained by carrying out high-throughput sequencing on the to-be-detected circulating tumor DNA and comprise a plurality of pairs of double-ended sequences; comparing the sequencing data with the reference genomic sequence to obtain a first comparing result, wherein the first comparing resultat least comprises genomic positions, base sequences and corresponding base mass value sequences of the double-ended sequences; and obtaining at least one consensus sequence and a base mass value sequence corresponding to each consensus sequence on the basis of the first comparing result. By means of the method disclosed by the invention, the technical problem that the accuracy is low when repeated sequence deleting or labeling is carried out on sample sequencing by adopting a sequencing data processing method in the prior art is solved.
Owner:GENE CRAB BIOTECH CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com