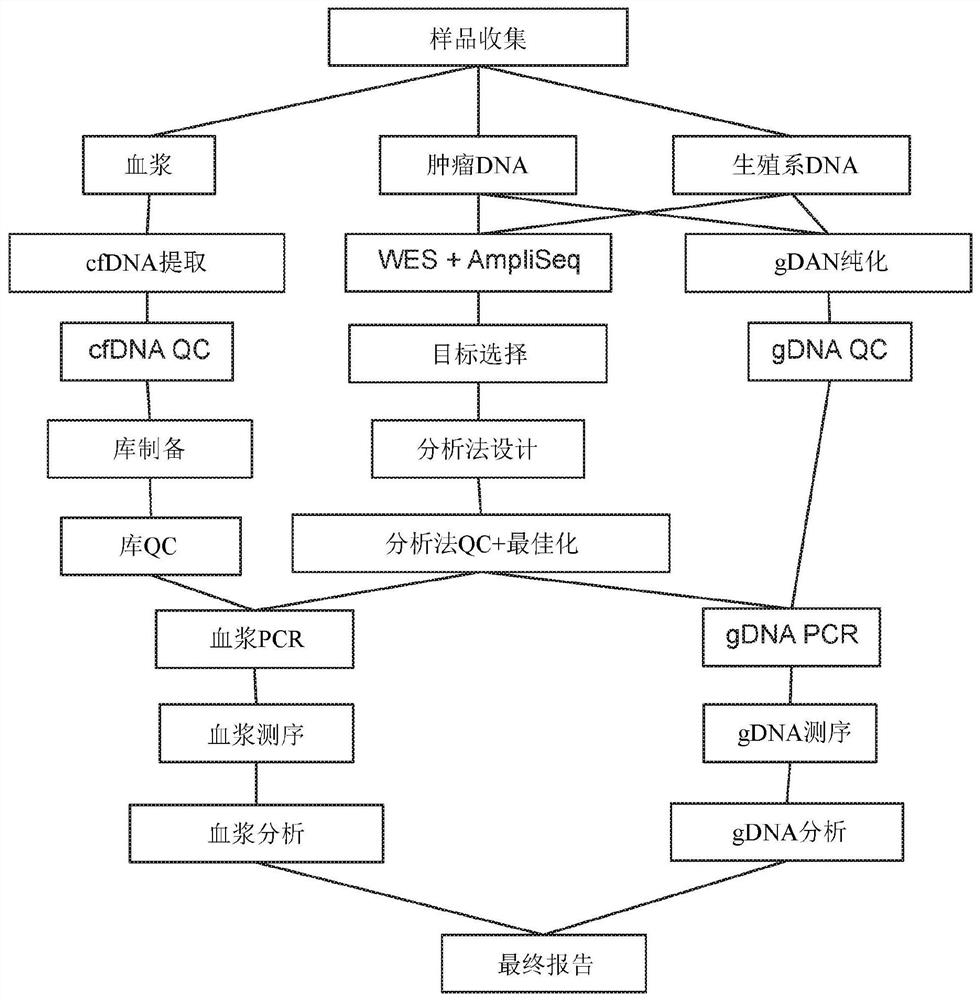
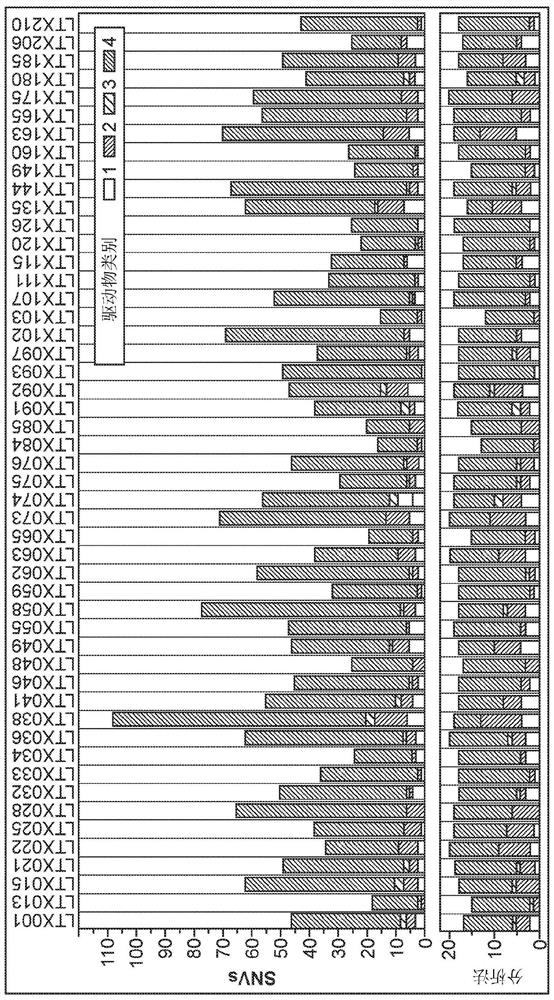
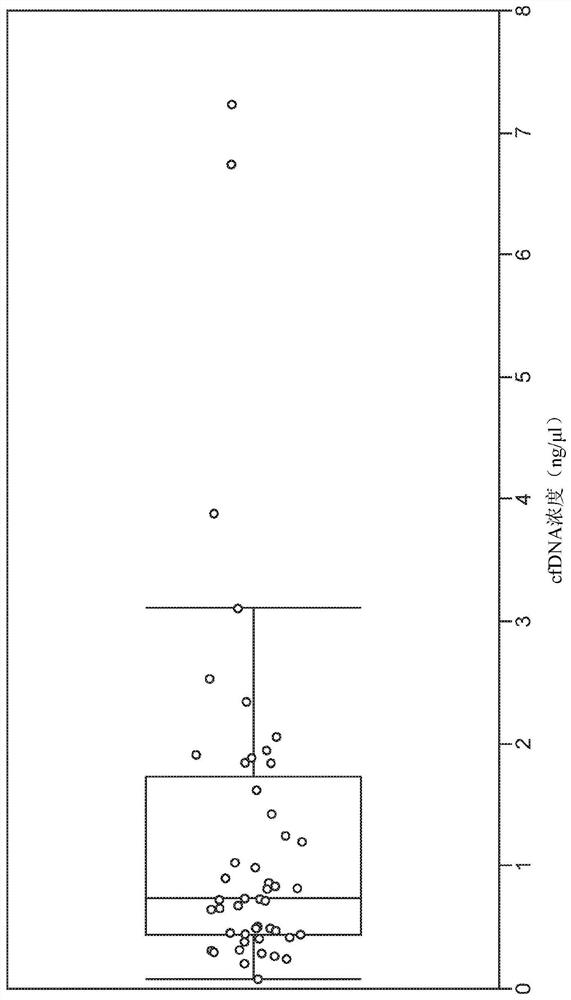
Methods for cancer detection and monitoring by means of personalized detection of circulating tumor DNA
A technology for cancer and breast cancer, applied in the direction of biochemical equipment and methods, microbial measurement/testing, etc., can solve problems such as insufficient sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0821] Example 1. Personalized circulating tumor DNA analysis for monitoring colorectal cancer
[0822] Early detection of disease recurrence has been shown to improve survival in colorectal cancer (CRC) patients. The detection of circulating tumor DNA (ctDNA) after surgery defines a subset of CRC patients at very high risk of recurrence. Previous studies have used small gene panel sequencing or digital droplet PCR for ctDNA analysis to monitor tumor burden in early CRC.
[0823] The aim of this example is to use a personalized multiplex PCR NGS platform targeting 16 tumor-specific mutations per patient to assess postoperative minimal residual disease and monitor treatment response in CRC.
[0824] 130 stage I-IV CRC patients treated with curative surgery and (optionally) adjuvant chemotherapy were included (see Table 1). Plasma samples were collected longitudinally at baseline before surgery and at planned control visits after surgery ( Figure 20A ). Whole-exome sequenci...
example 2
[0833] Example 2. Sequencing of plasma cfDNA from locally advanced bladder cancer patients for surveillance and efficacy monitoring
[0834] Studies of different cancer types have shown that circulating tumor DNA (ctDNA) content can be effectively used to monitor treatment response to neoadjuvant therapy and / or detect disease recurrence earlier than clinical and radiological detection. In bladder cancer, mutations in plasma have previously been used to monitor response during therapy and identify early signs of metastatic disease. Recently, longitudinal ctDNA testing in NSCLC patients was described and a personalized circulating tumor DNA (ctDNA) assay was developed (Signatera TM RUO).
[0835] The study goals were to detect metastatic recurrence, assess prognosis, and monitor treatment response in ctDNA from longitudinally collected plasma samples using patient-specific mutations identified in primary tumors.
[0836] Clinical protocol. Patients diagnosed with locally adva...
example 3
[0852] Example 3. Noninvasive Cancer Recurrence Detection and Therapy Monitoring Assay Based on Highly Sensitive Patient-Specific Multiplex PCR NGS
[0853] Identification of tumor mutations in circulating cell-free DNA has great potential for non-invasive detection of cancer recurrence prior to clinical manifestation, detection of minimal residual disease following curative-intent therapy, and detection of therapeutically relevant mutations. Review and report results of analytical validation of tumor-specific variant detection performed by the current version of the assay.
[0854] Customized monitoring technology RUO.Signatera for patients with solid tumors TM The (RUO) approach begins with the identification and prioritization of somatic mutations from whole-exome sequencing of tumor and matched normal samples. Patient-specific multiplex PCR assays targeting 16 somatic single-nucleotide and indel variants were then analyzed by massively parallel sequencing of plasma sample...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com