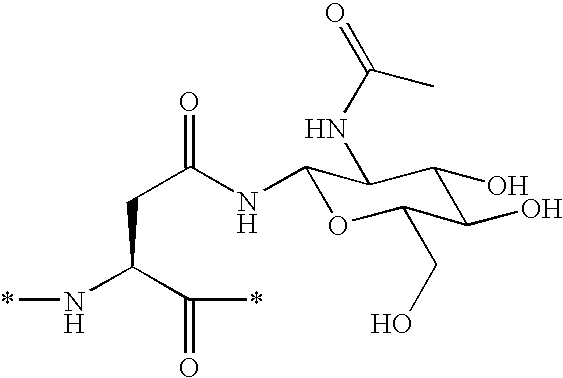
Patents
Literature
41 results about "Therapy monitoring" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Virion Derived Protein Nanoparticles For Delivering Radioisotopes For The Diagnosis And Treatment Of Malignant And Systemic Disease And The Monitoring Of Therapy
InactiveUS20130116408A1Precise deliveryImproved imaging differentiationVirus peptidesDepsipeptidesTherapy monitoringIsotope
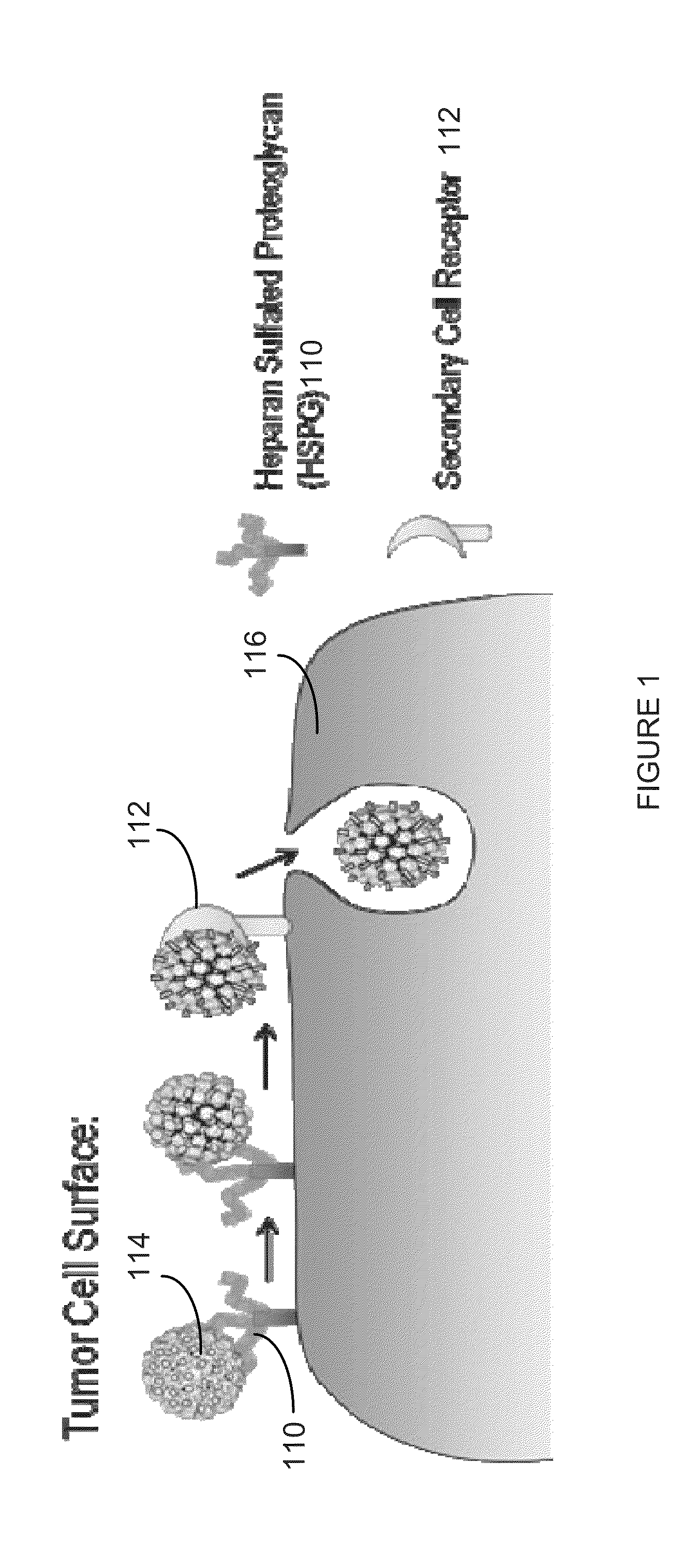
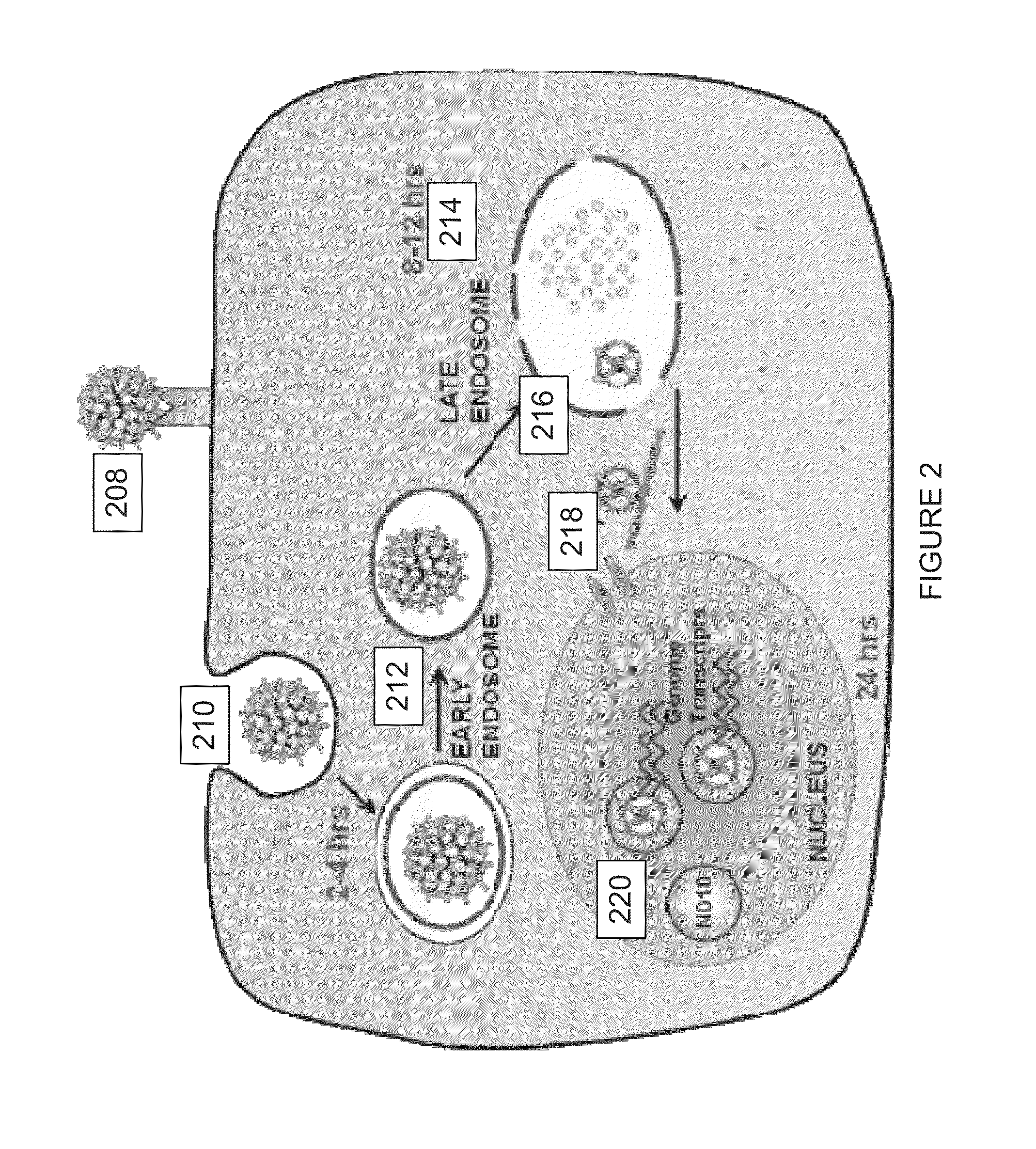
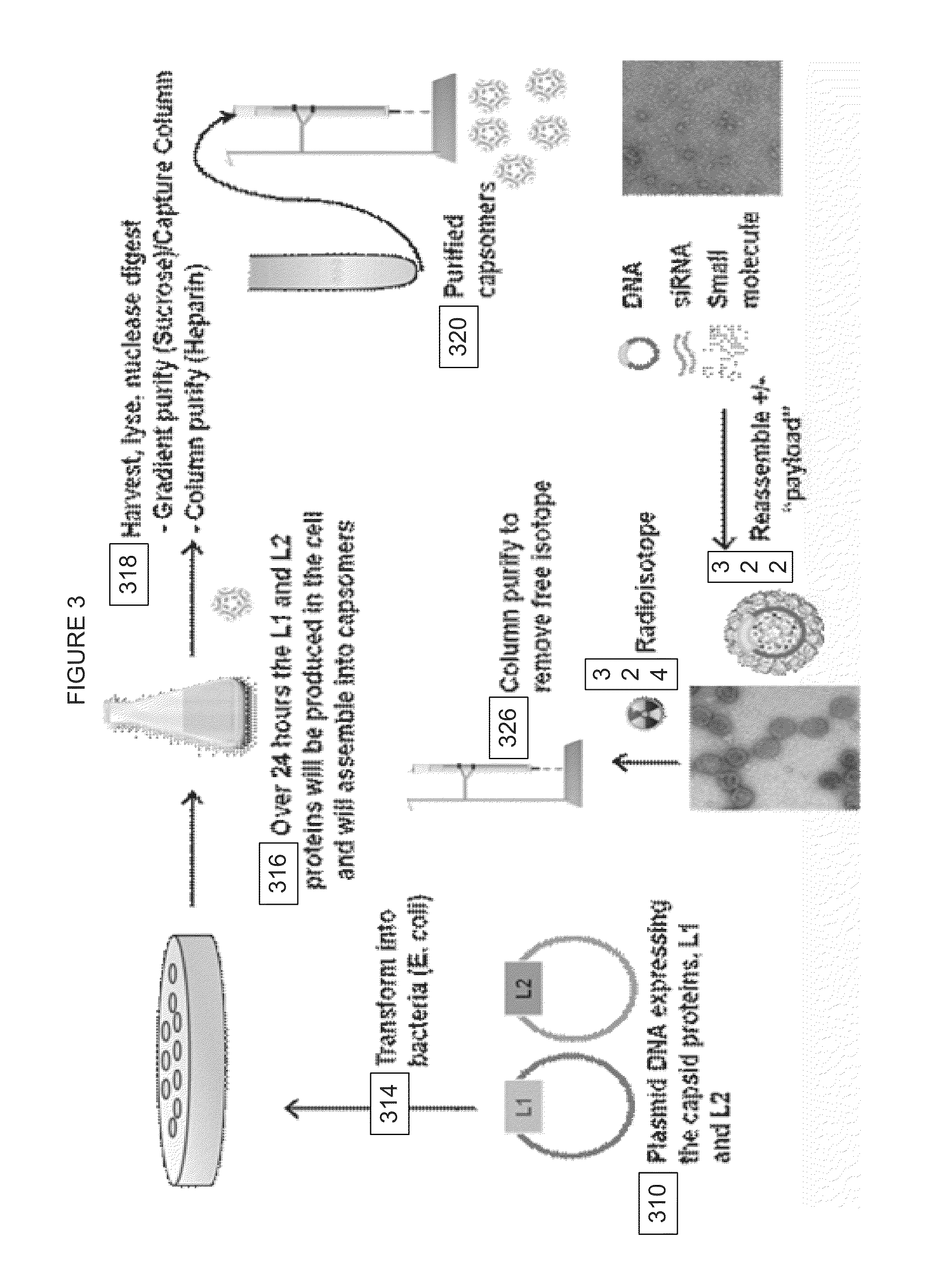
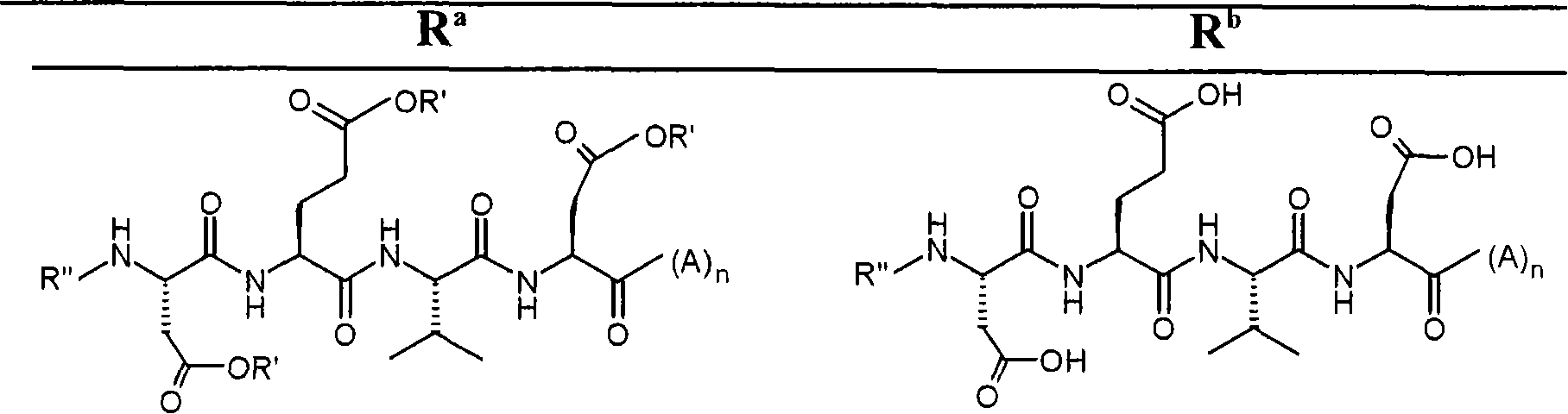
The invention is directed to novel compositions and methods utilizing virion derived protein nanoparticles for delivery of medical imaging agents and therapeutic agents for the diagnosis and treatment of malignant and systemic diseases. The nanoparticles of the present invention are designed to deliver radioactive isotopes suitable for imaging a tumor and its metastases. Additionally, the nanoparticles may deliver a radioisotope that is suitable for treating a tumor and its metastases by alpha, beta or gamma radiation. Alternatively, the virion derived nanoparticles may deliver a treatment agent for cancer or a combination of a radioisotope and a cancer treatment agent. Additionally the virion derived nanoparticle may include delivery of a drug that enhances the immune system's recognition of the tumor.
Owner:AURA BIOSCI
Methods, apparatus and articles-of-manufacture for noninvasive measurement and monitoring of peripheral blood flow, perfusion, cardiac output biophysic stress and cardiovascular condition
InactiveUS7192403B2Avoid flow turbulenceAvoid flow blood flow measurement distortionCatheterSensorsNon invasiveMulti sensor
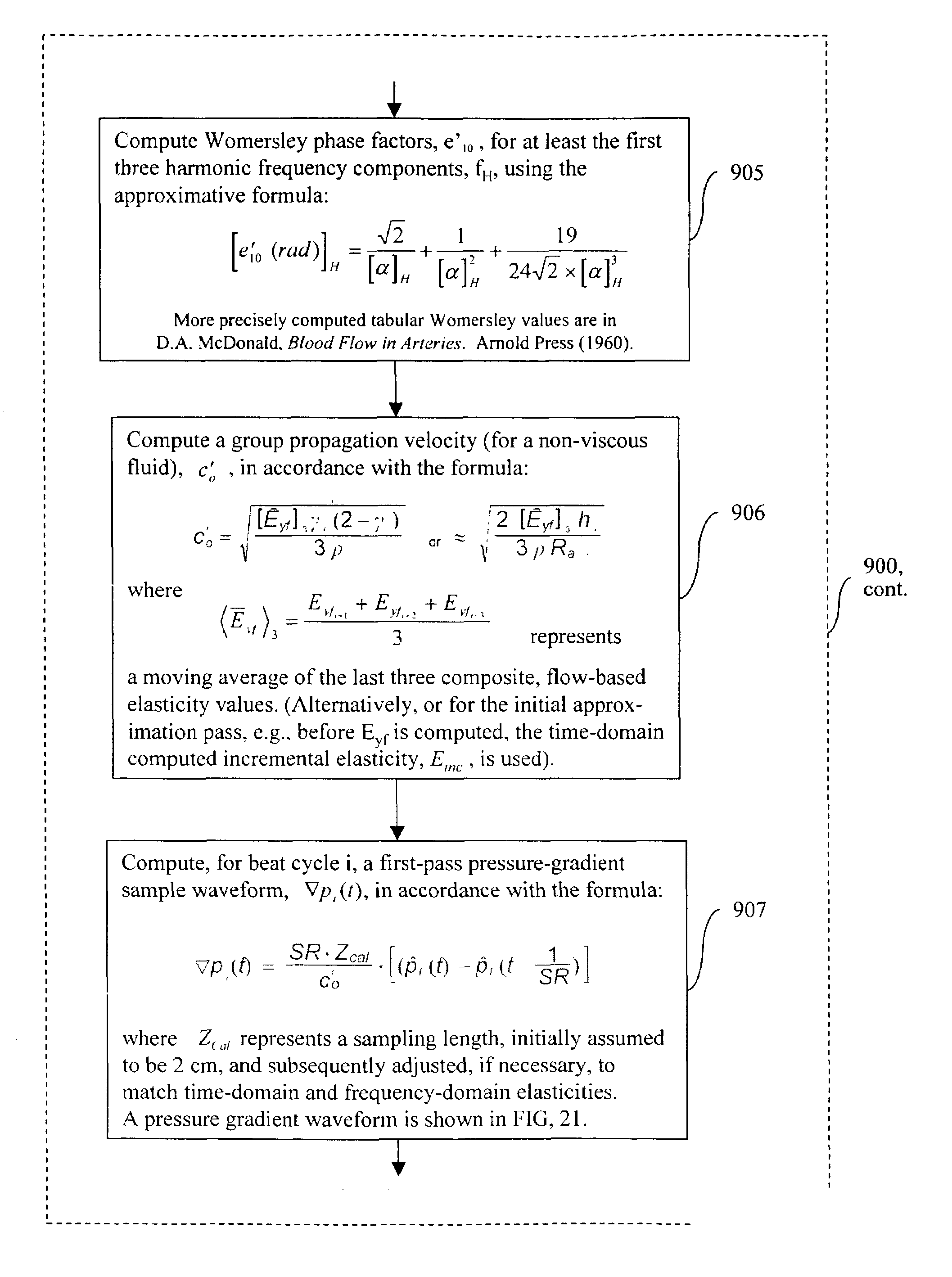
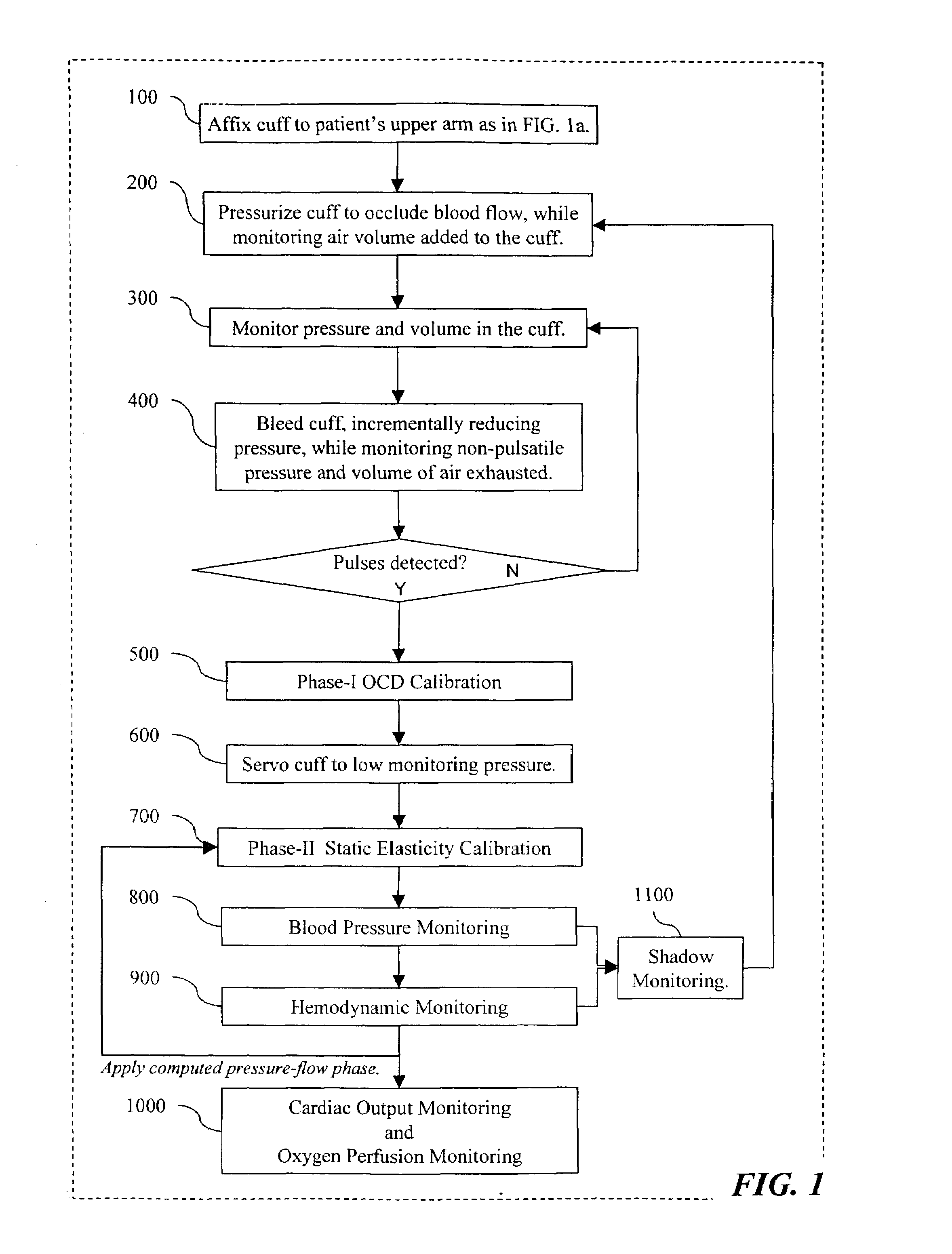
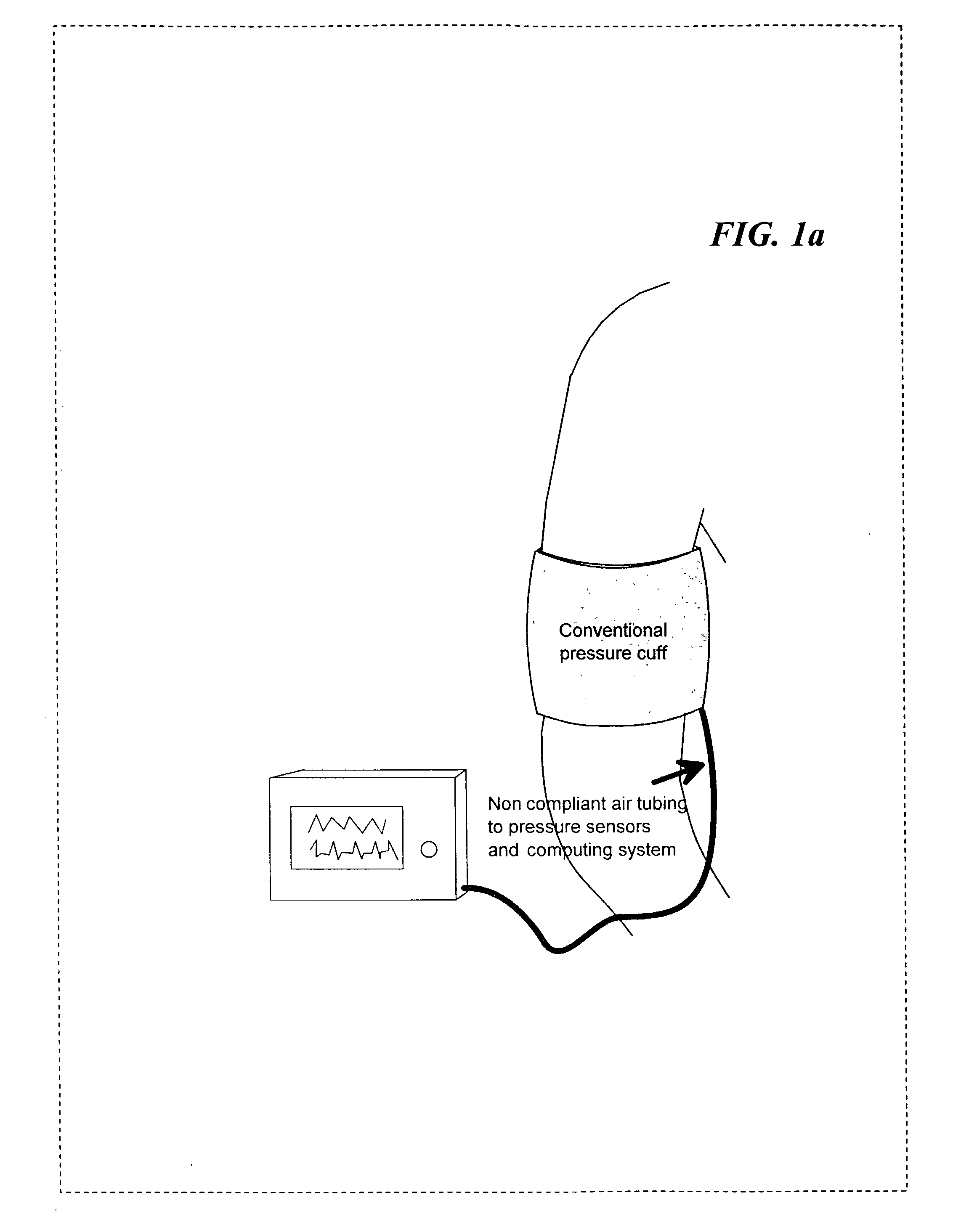
The invention relates to methods, apparatus, articles-of-manufacture, and coded data signals for measuring cardiac output, limb blood flow, perfusion, blood pressure, artery elasticity, and cardiovascular deterioration and disease, including performing these measurements on a continuous heart beat-by-beat basis, for humans and animals. Unlike empirical methods of other noninvasive blood pressure concepts, the invention is grounded on scientifically appropriate hemodynamic principles that studies have validated as accurate, and is practical for wide clinical use. Devices constructed in accordance with the invention can be comfortably employed for numerous applications, including hospital monitoring, physician's office cardiovascular disease management and drug therapy monitoring, home monitoring, and athletic applications.The invention may be implemented in a variety of single or multi-sensor embodiments, such as: invasive pressure cannula sensor systems; non invasive pressure transducer arrays and piezo or other strain sensing materials that are placed against the skin above arteries; “upstream” pulsing-sensors (that apply single or multi-frequency vibrations that are measured “downstream” from the first placement location); other types of plethysmographic sensors; sonic / ultrasonic / Doppler sensors; MRI blood spin magnetizer / sensors; oxygen sensors; and electrocardiographic sensors, etc.
Owner:RUSSELL TED W
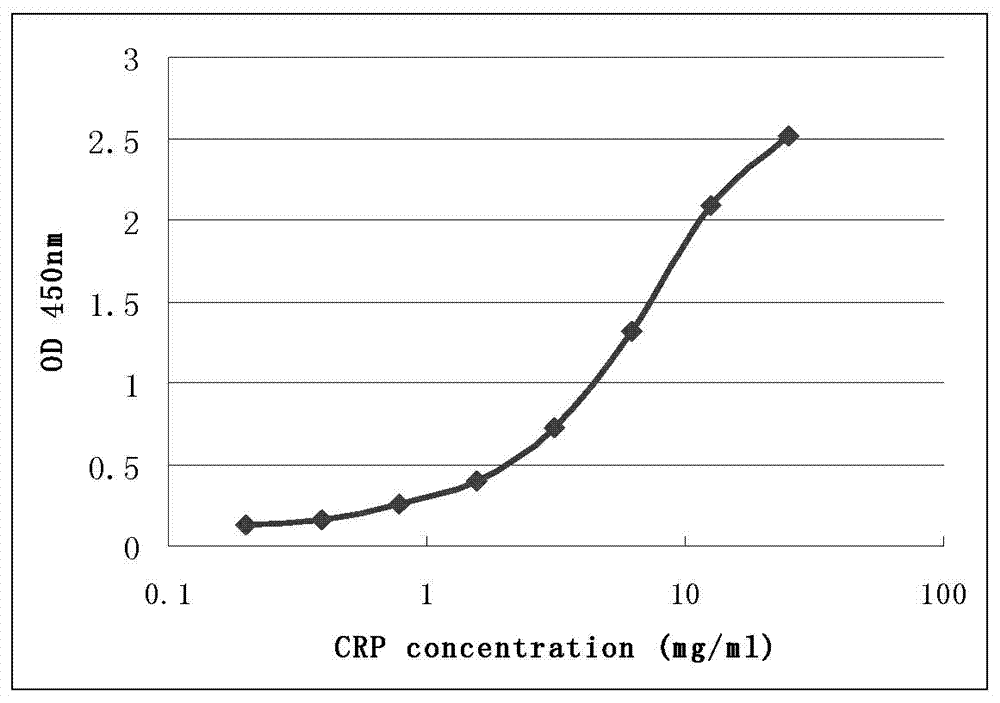
CRP monoclonal antibody nanometer latex microsphere composition and preparation process thereof
ActiveCN103073642ASmall variance between different production batchesQuality improvementImmunoglobulins against animals/humansCarrier-bound/immobilised peptidesTherapy monitoringMicrosphere
The present invention discloses a CRP monoclonal antibody, a CRP antibody nanometer latex microsphere composition and a preparation process thereof. According to the CRP antibody nanometer latex microsphere composition, CRP monoclonal antibodies with different epitopes and carboxylated polystyrene microspheres with different particle sizes are subjected to covalent cross-linking to form conjugates, and then the different conjugates are mixed according to a certain ratio to prepare the CRP monoclonal antibody nanometer latex microsphere composition. The CRP monoclonal antibody nanometer latex microsphere composition can be applicable for automatic biochemical analyzers and special protein analyzers, can be provided for full measuring range determination of C-reactive protein concentration in human whole blood and serum, and can be used in differential diagnosis of bacterial infections and viral infections, drug therapy monitoring and cardiovascular disease risk assessments.
Owner:深圳伯美生物医药有限公司
Novel imaging agents
InactiveUS20060275215A1Useful imageAntibacterial agentsUltrasonic/sonic/infrasonic diagnosticsTherapy monitoringCaspase 3
The present invention relates to diagnostic imaging agents for in vivo imaging. The imaging agents comprise a synthetic caspase-3 inhibitor labelled with an imaging moiety suitable for diagnostic imaging in vivo. The invention also provides pharmaceutical and radiopharmaceutical compositions comprising the imaging agents, together with kits for the preparation of the radiopharmaceuticals. Also described are chelator conjugates of the caspase-3 inhibitor, which are suitable for the preparation of imaging agents comprising a radioactive or paramagnetic metal ion. The imaging agents are useful for the diagnostic imaging and or therapy monitoring in vivo of various disease states where caspase-3 is involved.
Owner:HISCOCK DUNCAN +2
Methods and compositions for identifying target cell cytolytic lymphocytes in a sample
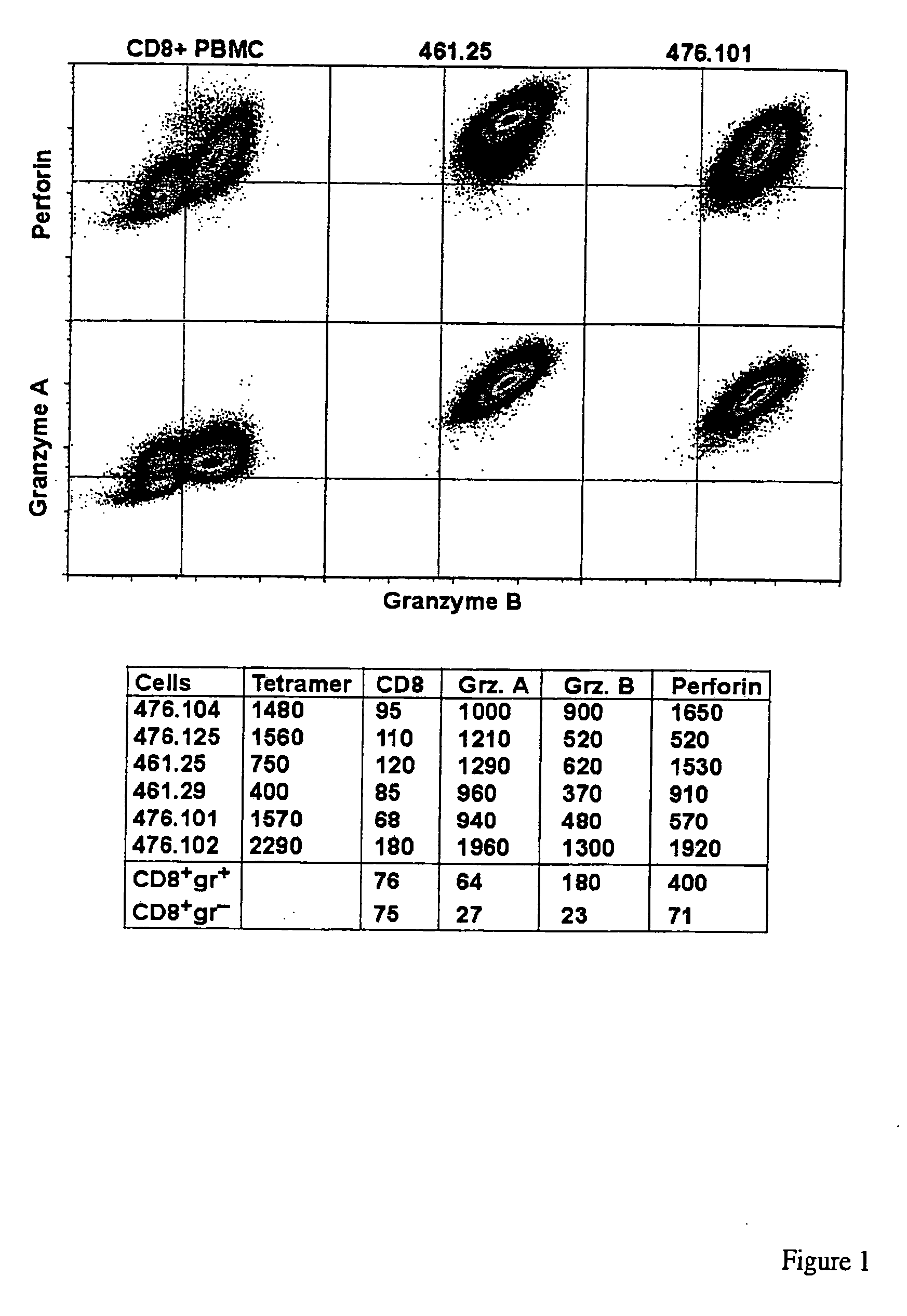
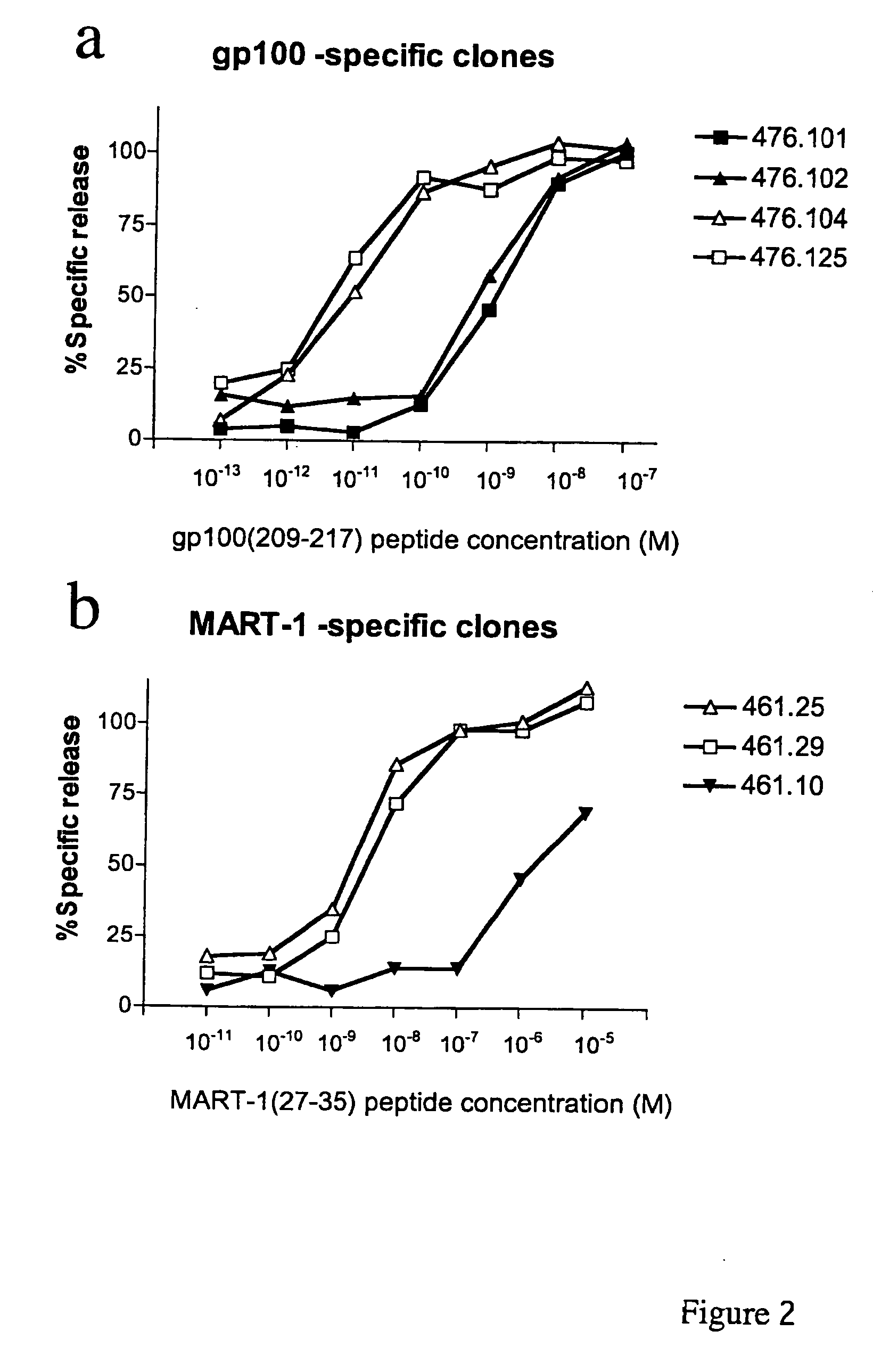
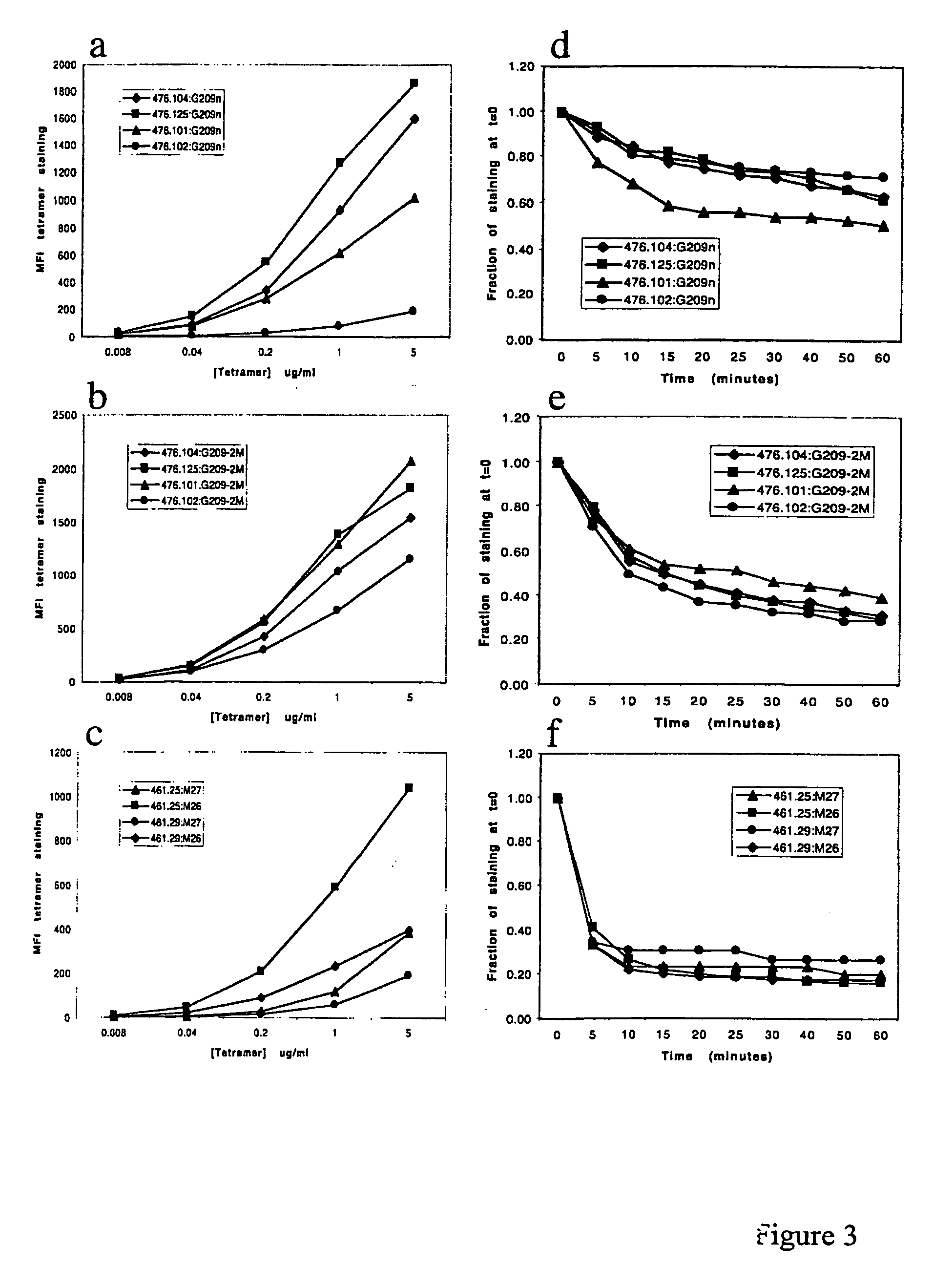
Methods and compositions for identifying target cell cytolytic lymphocytes, e.g., T-cells, such as neoplastic cell cytolytic T-cells, in a subject are provided. In practicing the subject methods, the sample is contacted with a target cell stimulator, e.g., a neoplastic cell, and a detectably labeled granule membrane protein specific binding agent. Following contact, any resultant labeled lymphocytes, e.g., T-cells, are identified as lymphocytes cytolytic for the target cell. Also provided are compositions, kits, and systems for practicing the subject methods. The subject methods find use in a variety of different applications, including disease / therapy monitoring applications and therapeutic applications.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Proteomic profiling method useful for condition diagnosis and monitoring, composition screening, and therapeutic monitoring
InactiveUS20080172184A1Low costLower levelCompound screeningApoptosis detectionTherapy monitoringProtein composition
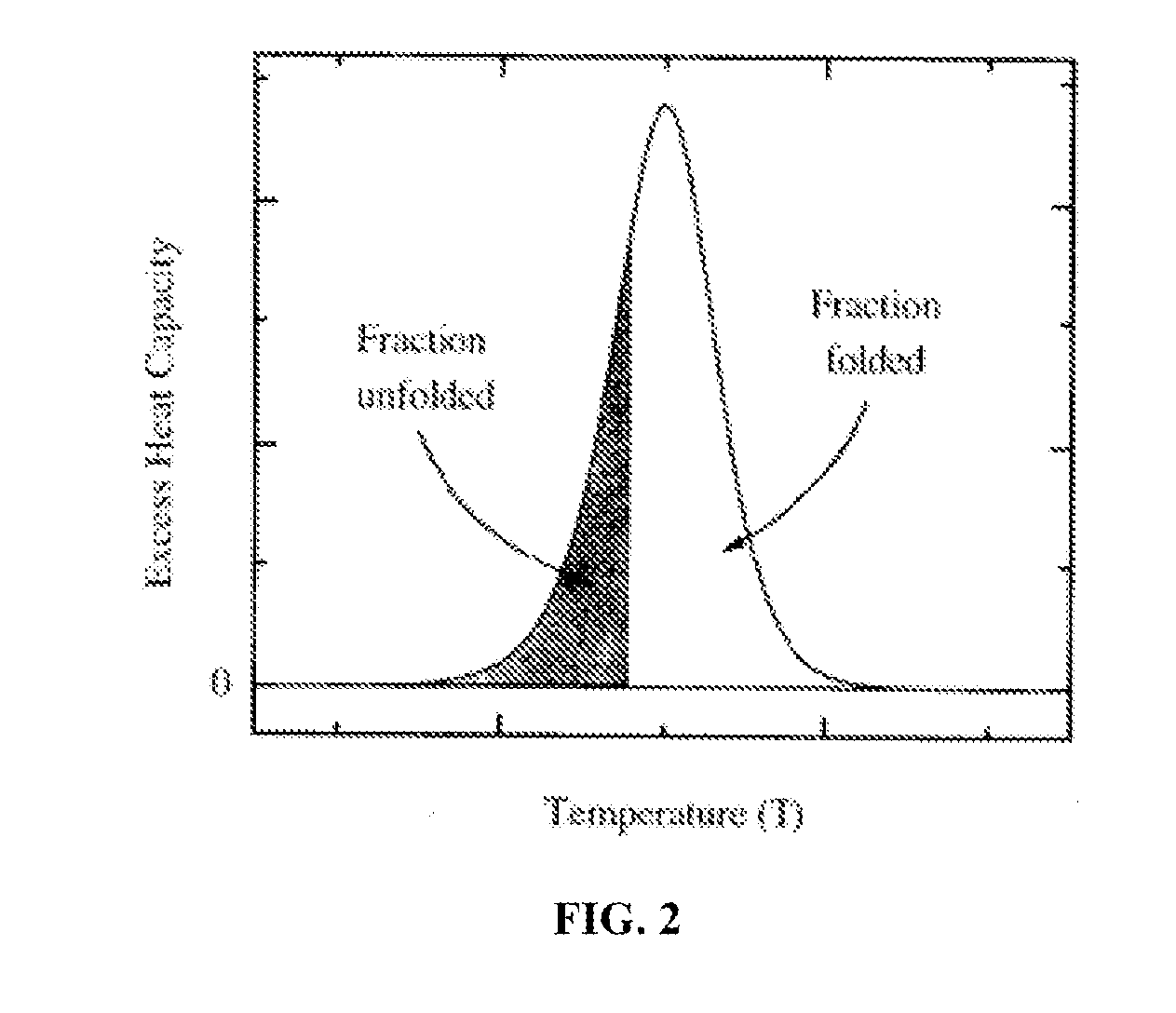
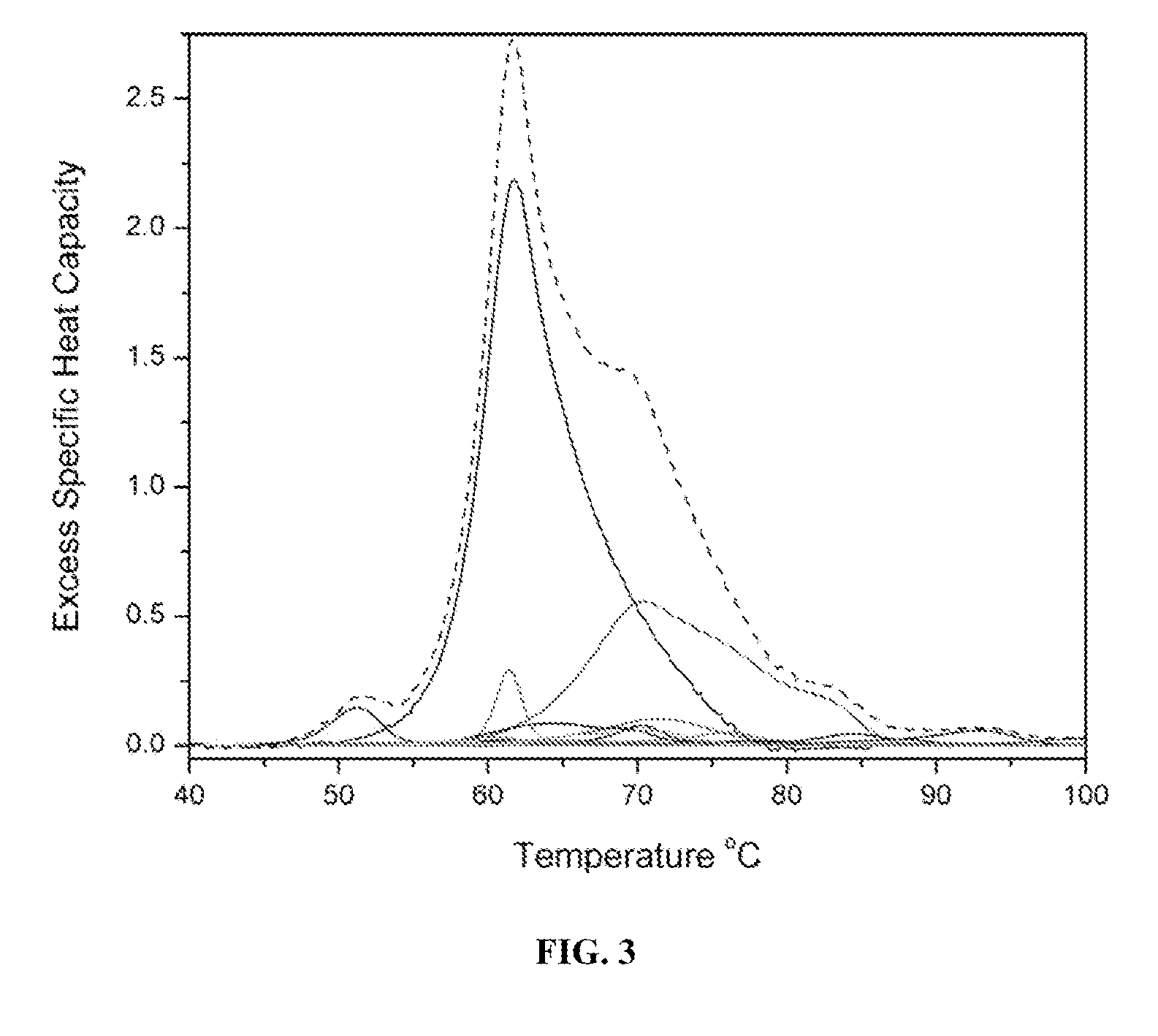
A method of diagnosing or monitoring a condition of interest in a subject includes comparing thermograms generated using differential scanning calorimetery. A signature thermogram contains a protein composition pattern for a sample obtained from the subject. The signature thermogram is compared to a standard thermogram. Standard thermograms can include a negative standard thermogram containing a protein composition pattern associated with an absence of the condition of interest, and a positive standard thermogram containing a protein composition pattern associated with a presence of the condition of interest.
Owner:UNIV OF LOUISVILLE RES FOUND INC
Circulating tumor cell separation device adopting combined field flow separation
ActiveCN105647799AHigh selectivityProcessing speedBiological substance pretreatmentsStress based microorganism growth stimulationTherapy monitoringDisease
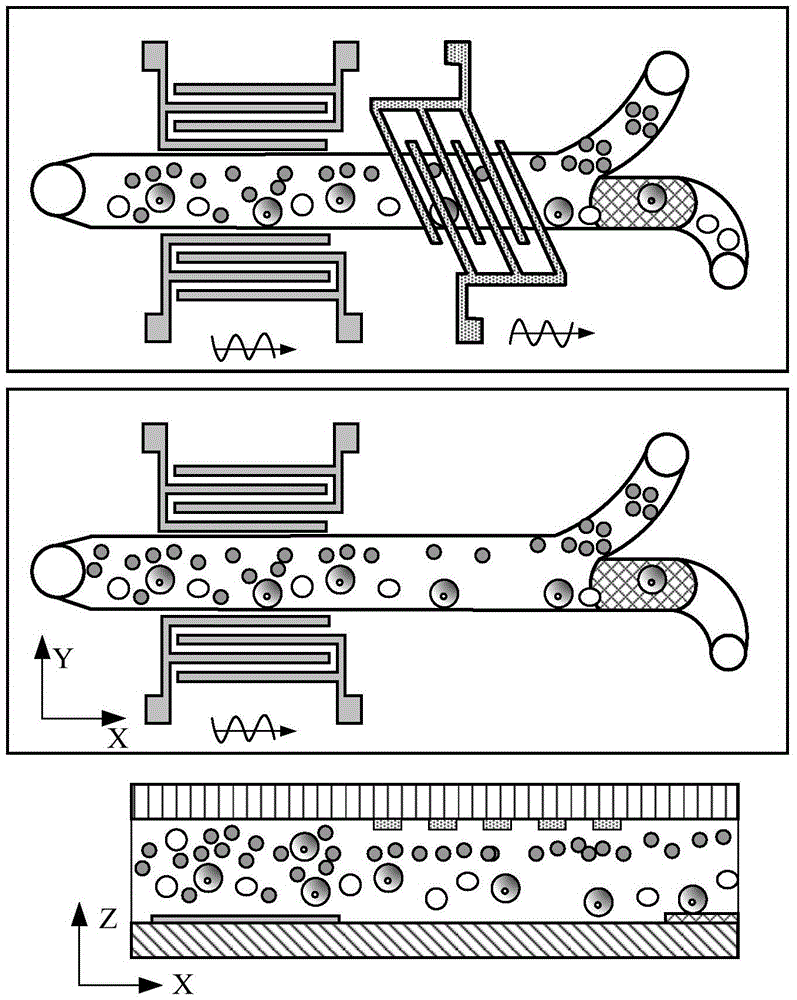
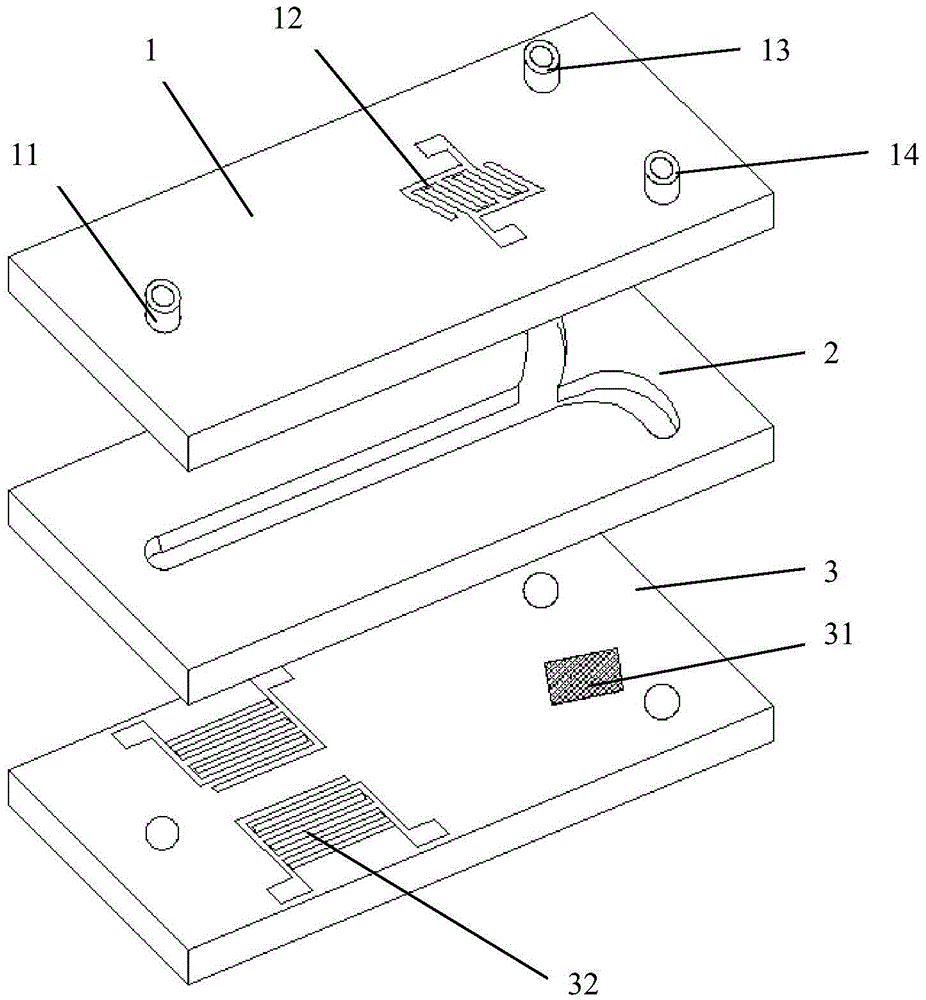
The invention provides a circulating tumor cell separation device adopting combined field flow separation. The circulating tumor cell separation device comprises an upper cover plate, a channel plate and a substrate plate from top to bottom; a two-step separation method comprising the field flow separation and specific molecular affinity identification is adopted, wherein the field flow separation adopts an acoustic surface wave driving mode and a dielectrophoresis driving mode, horizontal cell grouping migration and cell layering in a vertical plane are realized respectively, and erythrocytes are preliminarily separated from other cell populations; a residual leucocyte mixed liquid passes through a cell recognition area, circulating tumor cells are fixed in the recognition area by recognition ligands, and a tumor cell sample is acquired through releasing operation finally. The device can rapidly realize circulating tumor cell separation without marks, has great significance in the aspects of patient's disease diagnosis, therapy monitoring, pathological research and the like, and can be used for a separation process of other cells, macromolecular protein and micro-particles.
Owner:上海揽微赛尔生物科技有限公司
Kit for rapidly detecting nucleic acid of hepatitis C virus and detection method of kit
ActiveCN107022651AHigh precisionIncrease costMicrobiological testing/measurementTherapy monitoringPositive control
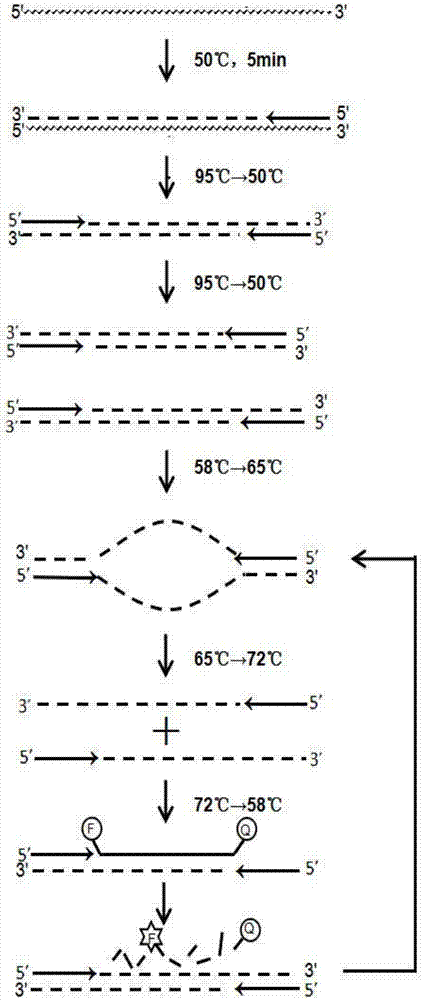
The invention discloses a kit for rapidly detecting nucleic acid of a hepatitis C virus (HCV) by a one-step method. The kit comprises a nucleic acid extracting solution, an RT-qPCR reaction solution, a negative control, a positive control and a positive reference. The kit is mainly used for overcoming the disadvantage of the existing kit on the market at present that the time for nucleic acid detection is relatively long. According to the kit, the denaturation of the nucleic acid at a relatively low temperature is achieved through changing ingredients of a reaction buffer and uniquely designing and synthesizing primers and probes on the basis of the conventional RT-qPCR basic reaction principle, then, the temperature difference of denaturation and renaturation during PCR is reduced, and finally, whole reaction time is greatly shortened. The kit disclosed by the invention has relatively high detection sensitivity, specificity and repeatability and can be used for detecting common six genotypes of the hepatitis C virus; meanwhile, the kit is also applicable to the conventional quantitative PCR instrument, so that an accurate basis is provided for auxiliary diagnosis of hepatitis C virus infections and drug therapy monitoring of infected persons.
Owner:辽宁润基生物科技有限公司
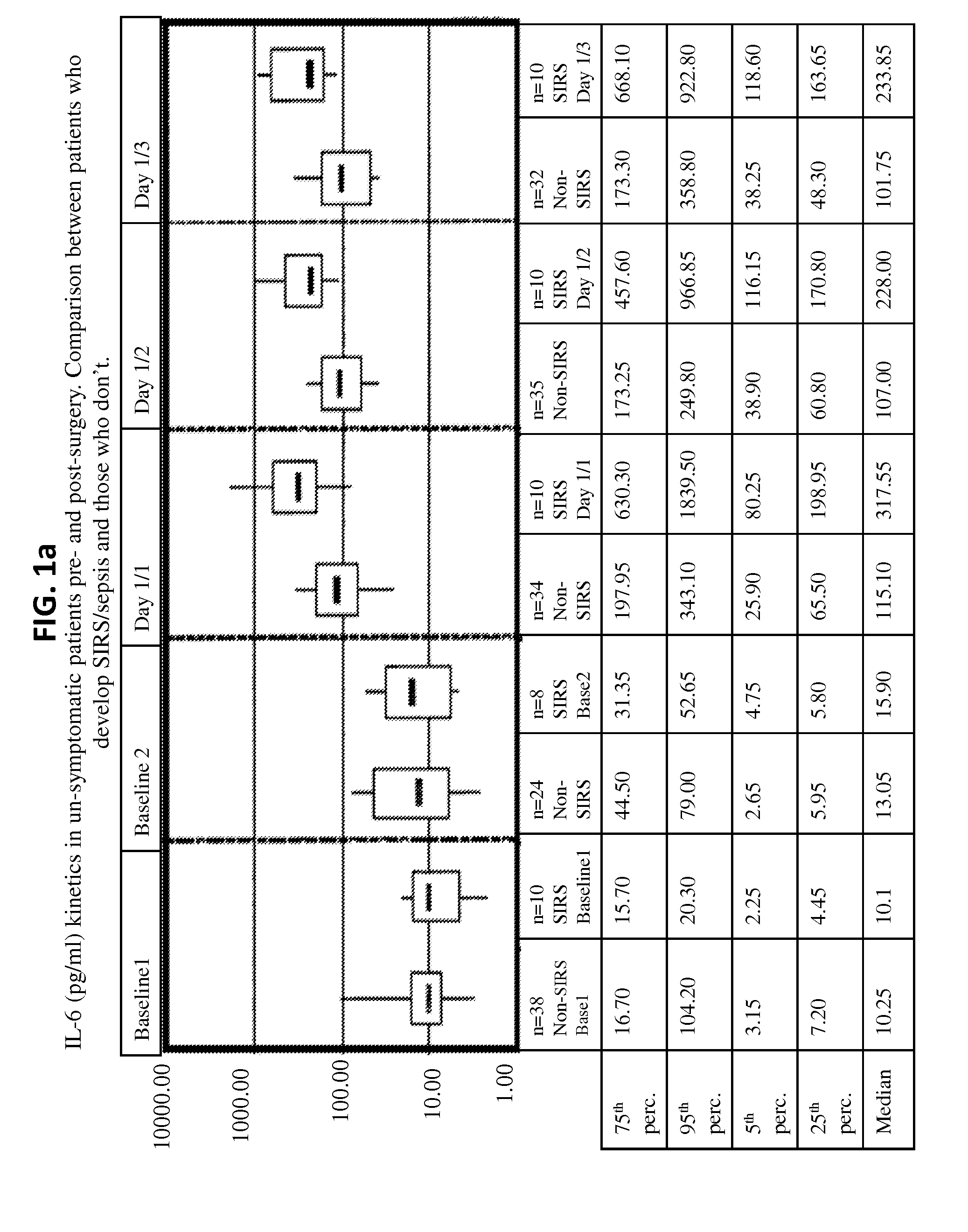
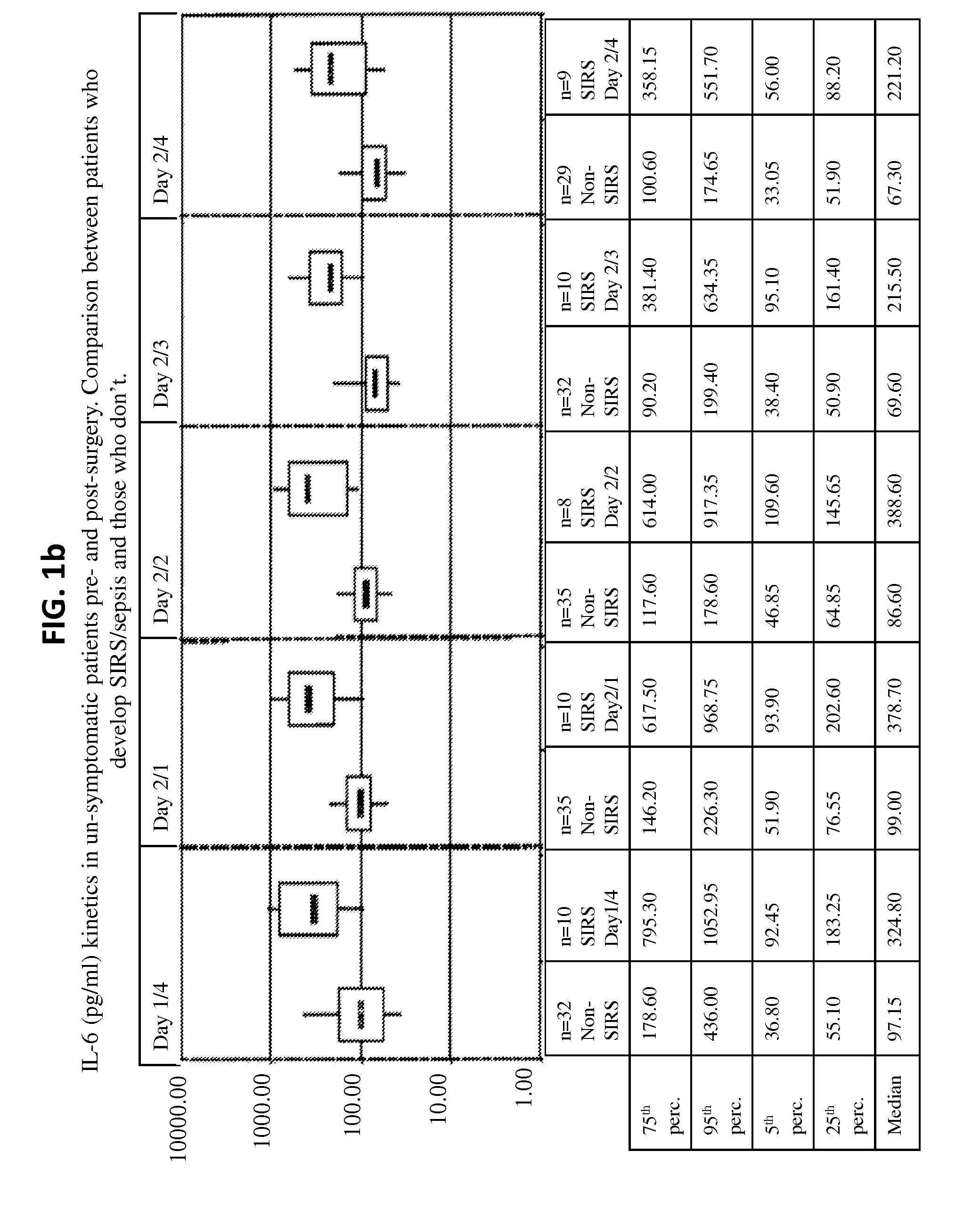
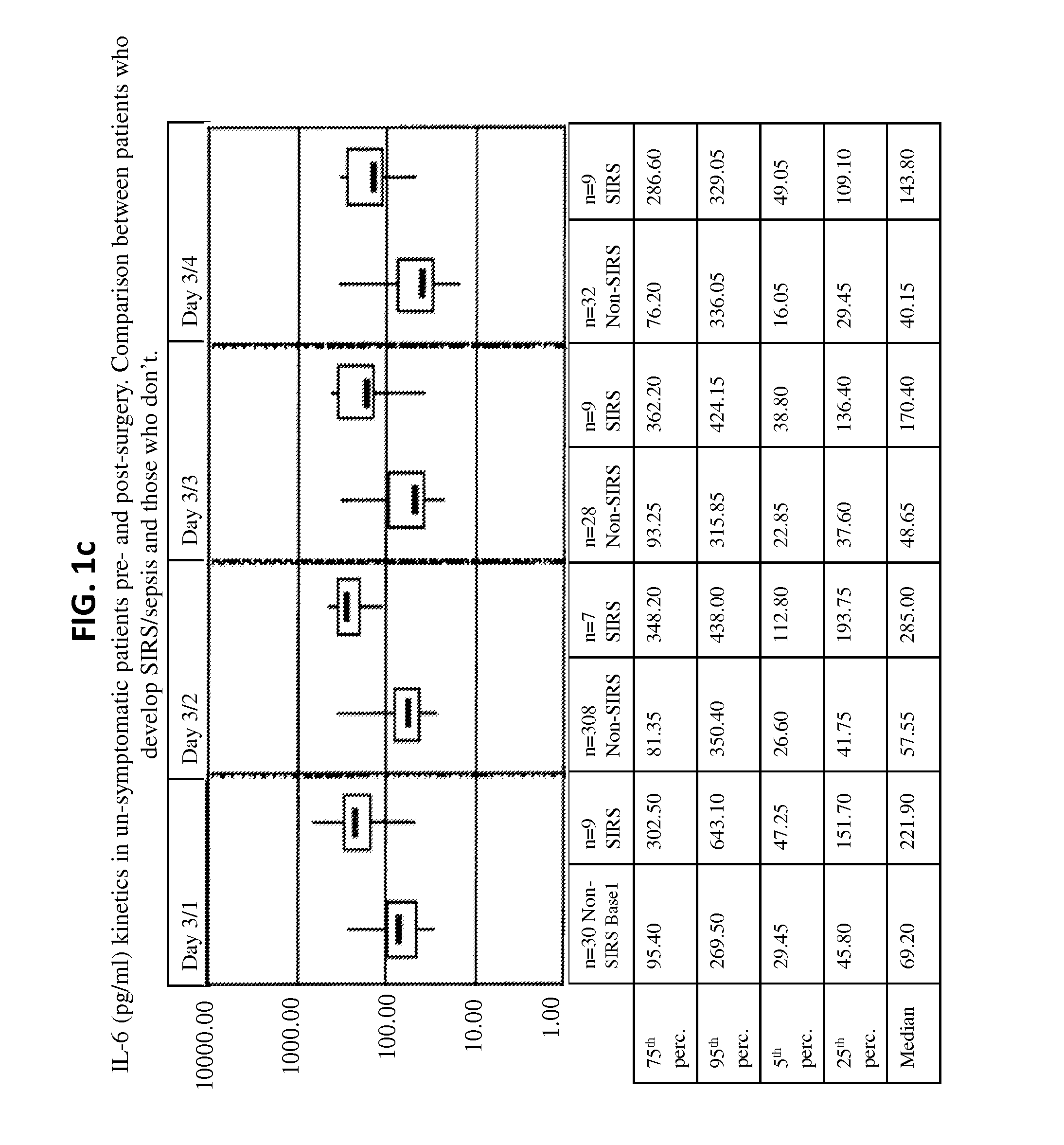
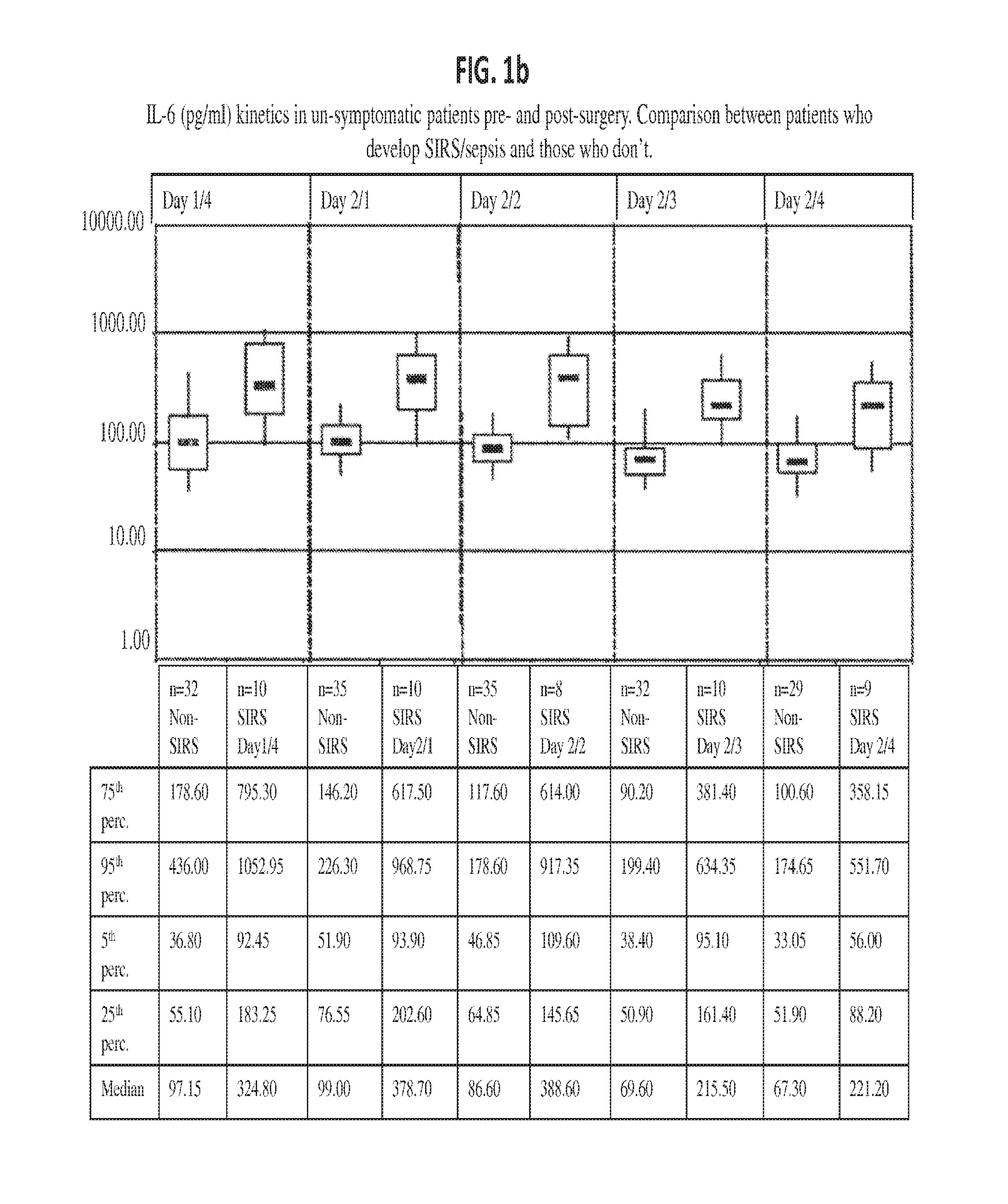
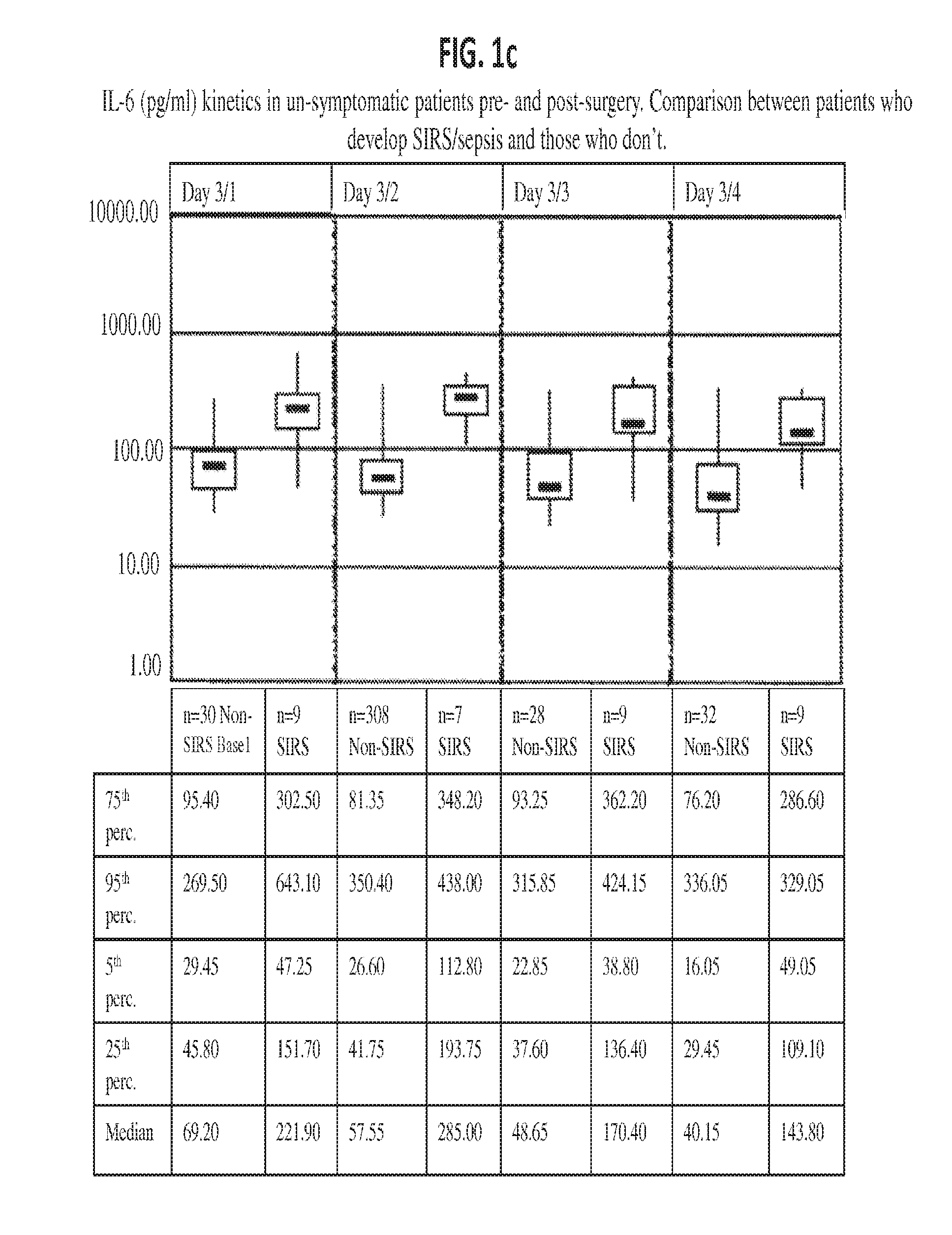
Il-6 detection based early diagnosis and prediction of systematic inflammatory response syndrome and sepsis in asymptomatic patients
InactiveUS20130052671A1Chemiluminescene/bioluminescenceDisease diagnosisSurgical operationTherapy monitoring
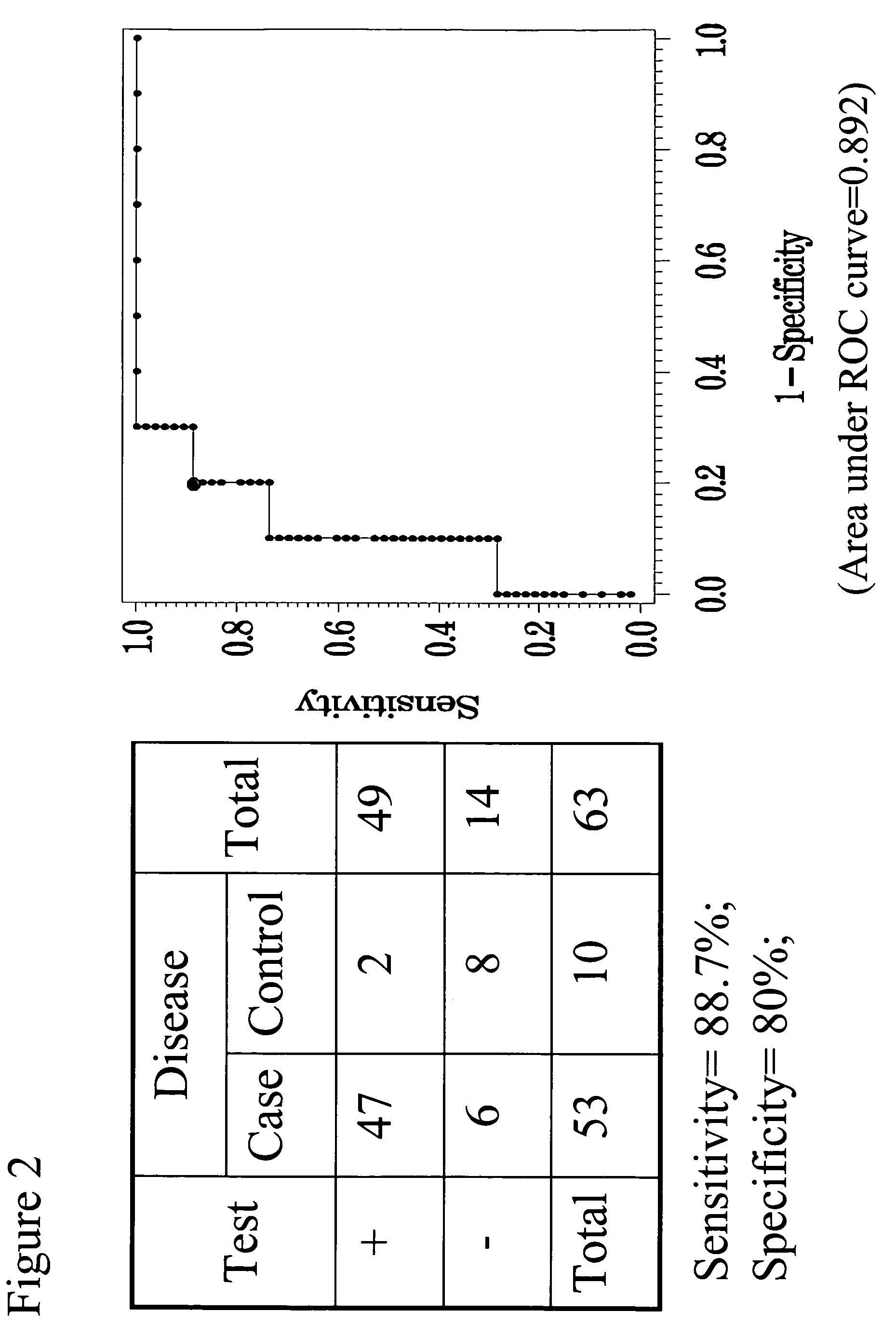
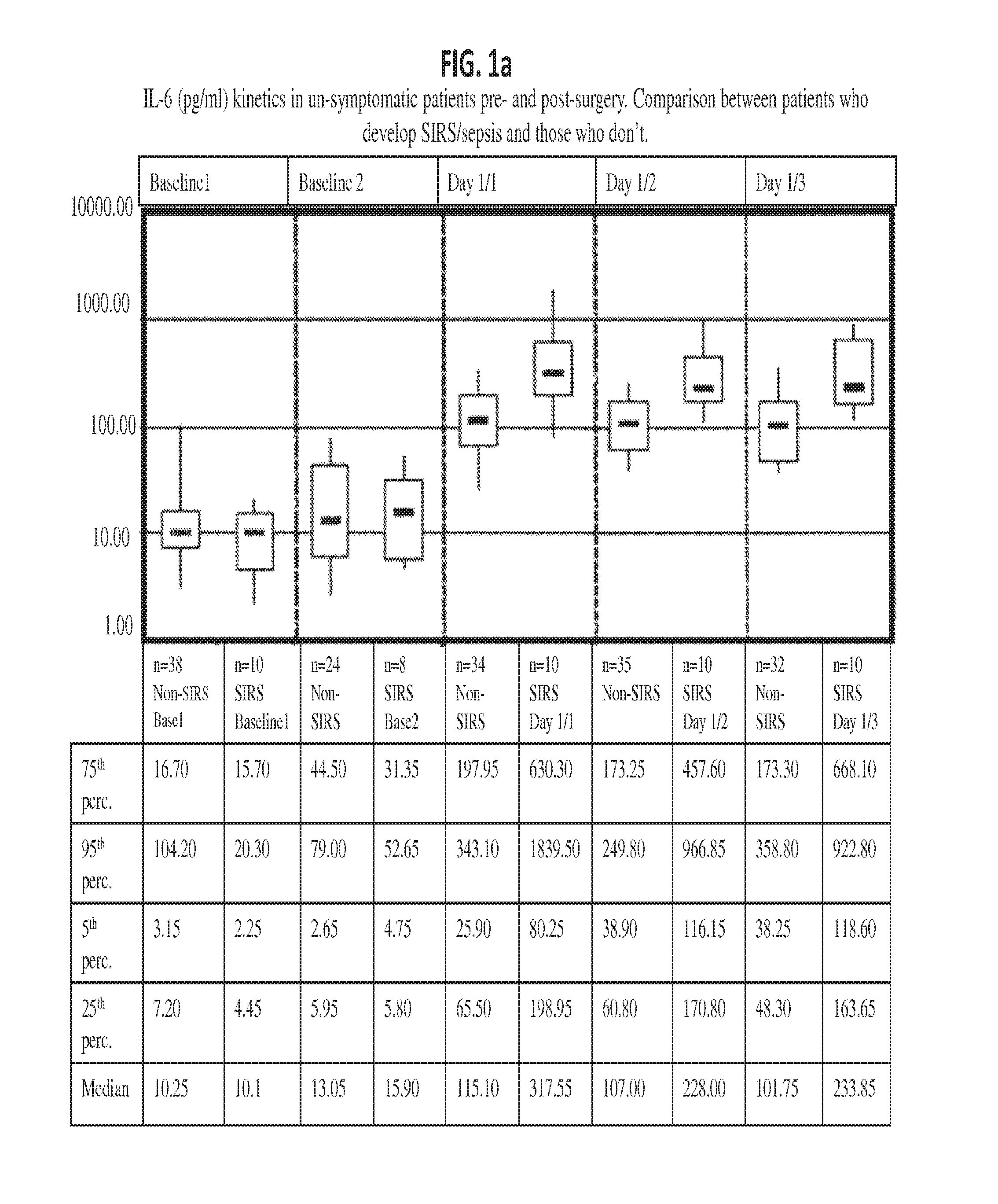
Methods, systems and kits for the early diagnosis or prediction of systemic inflammatory response syndrome (SIRS) including sepsis in asymptomatic patients, such as patients undergoing a surgical intervention, are provided. Some embodiments include a method and system for the detection or diagnosis of SIRS, or detection or diagnosis of a risk to suffer from or develop SIRS, in an asymptomatic patient comprising the steps of determining the level of IL-6 (or a variant thereof) in a sample from the patient; comparing the level of IL-6 (or a variant thereof) to a reference level; detecting or diagnosing SIRS or diagnosing a risk to suffer from or develop SIRS, wherein the sample is isolated at least 2 times at short intervals and the determining and comparing steps are both repeated for each sample. Also provided are methods, systems and kits for therapy monitoring and mortality prediction.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC +1
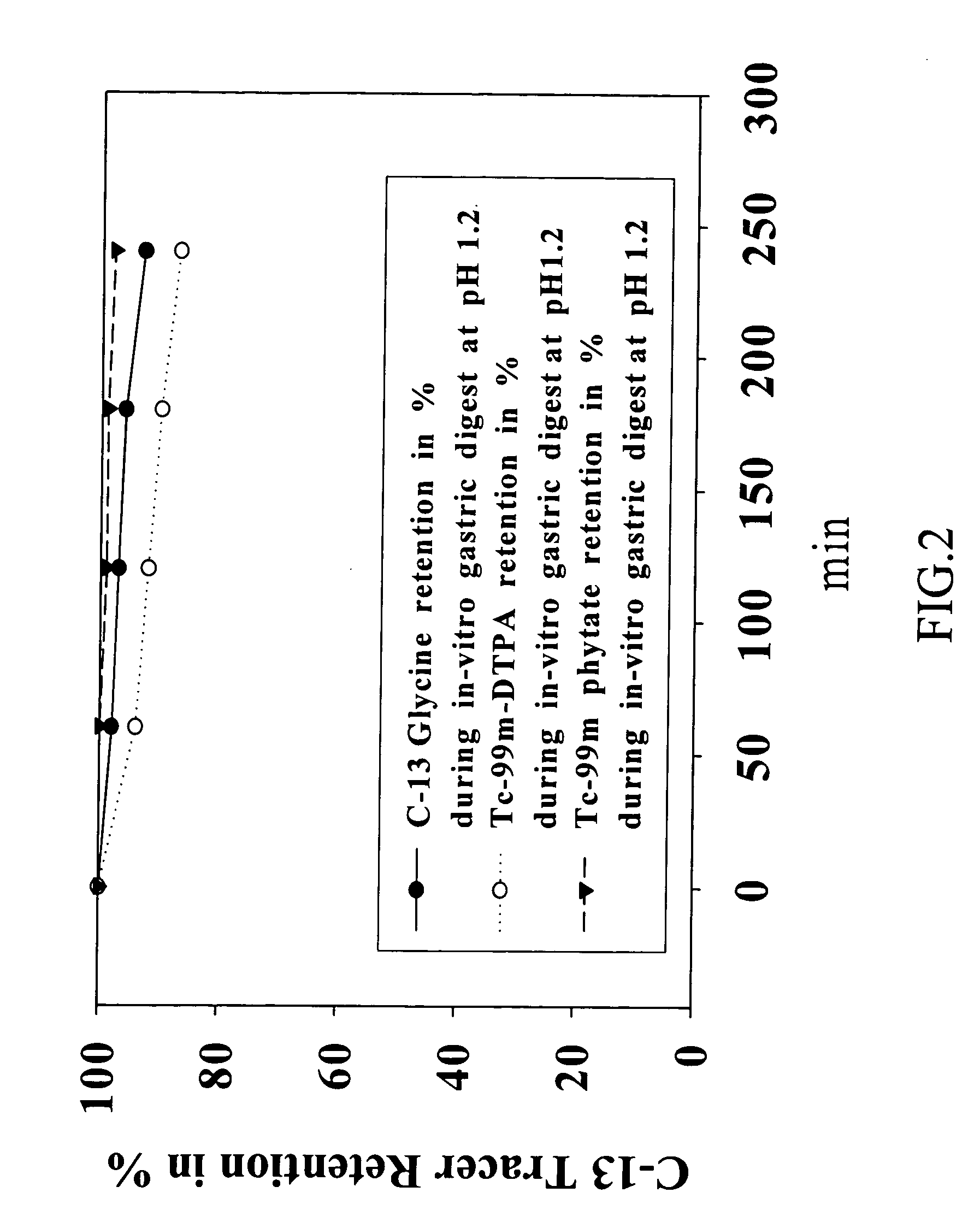
Kits for gastric emptying measurement
InactiveUS20070014718A1Small molecular weightQuick measurementCompounds screening/testingIn-vivo radioactive preparationsTherapy monitoringDisease
The present invention provides a test meal kits that are used in the diagnosis of gastrointestinal disorders characterized by changes in the rate of gastric emptying; and, with a breath test or a nuclear scintigraphy scan, are used to measure a half-gastric emptying time useful for therapy monitoring of gastrointestinal disorders in clinical.
Owner:INST NUCLEAR ENERGY RES ROCAEC
Insomnia-disorder psychotherapy system and method based on immersive VR
InactiveCN109646784AEasy accessMeet interaction needsDiagnostic recording/measuringSensorsTherapy monitoringTreatment effect
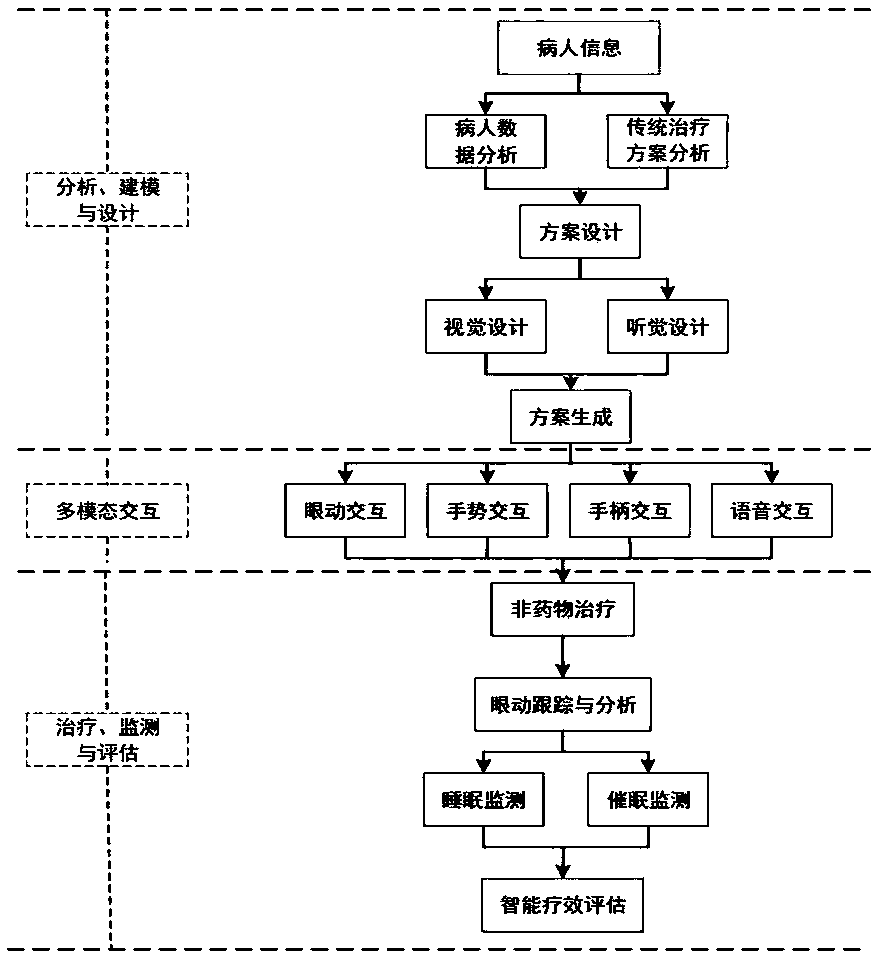
The invention provides an insomnia-disorder psychotherapy system based on immersive VR. By wearing a virtual reality helmet provided with an eye tracker, a user enters an insomnia therapy scene in aninteractive mode to receive insomnia therapy, and the insomnia therapy is intelligently detected and evaluated. Patients can select one or more modes of handle interaction, voice interaction, gestureinteraction, eye movement interaction and the like according to interaction habits of the patients and enter preferred safe scenes, and the interaction requirements of different patients are met; accurate therapy for the patients can be achieved through a multimode interaction mode, the matching degree in the therapy process can be increased to a certain degree, the patients can more rapidly get into the hypnotic state, and the therapy effect can be improved; intelligent therapy monitoring and automatic therapeutic-effect evaluation are achieved in the therapy process; the disadvantage that inthe therapy process, as the immersive VR helmet is worn, a psychological counselor cannot directly evaluate the therapeutic effect is effectively solved.
Owner:EAST CHINA INST OF COMPUTING TECH
Negative pressure wound therapy system based on external terminal monitoring
InactiveCN102805894ARealize remote controlRealize intelligent treatment monitoringWound drainsTransmissionTherapy monitoringWound therapy
The invention discloses a negative pressure wound therapy system based on external terminal monitoring. The negative pressure wound therapy system based on external terminal monitoring comprises a liquid collection bottle, a negative pressure wound therapy device, a data communication module and a terminal processing module. The liquid collection bottle is used for collecting wound fluid. The negative pressure wound therapy device is provided with a negative pressure source and a controller. The data communication module is used for information transceiving between the negative pressure wound therapy device and an external terminal device. The terminal processing module is used for processing information received from the negative pressure wound therapy device and monitoring therapy conditions of the negative pressure wound therapy device. The data communication module is available for data transceiving between the wound therapy device and the external terminal device. The terminal processing module is available for processing the information received from the wound therapy device and monitoring and controlling therapy of the wound therapy device, so that the negative pressure wound therapy system can process therapy data on the external terminal, which the system fails to process, and remote control and one to one, one-to-many or many-to-one intelligent therapy monitoring can be realized for medical staff.
Owner:FORYOU MEDICAL ELECTRONICS
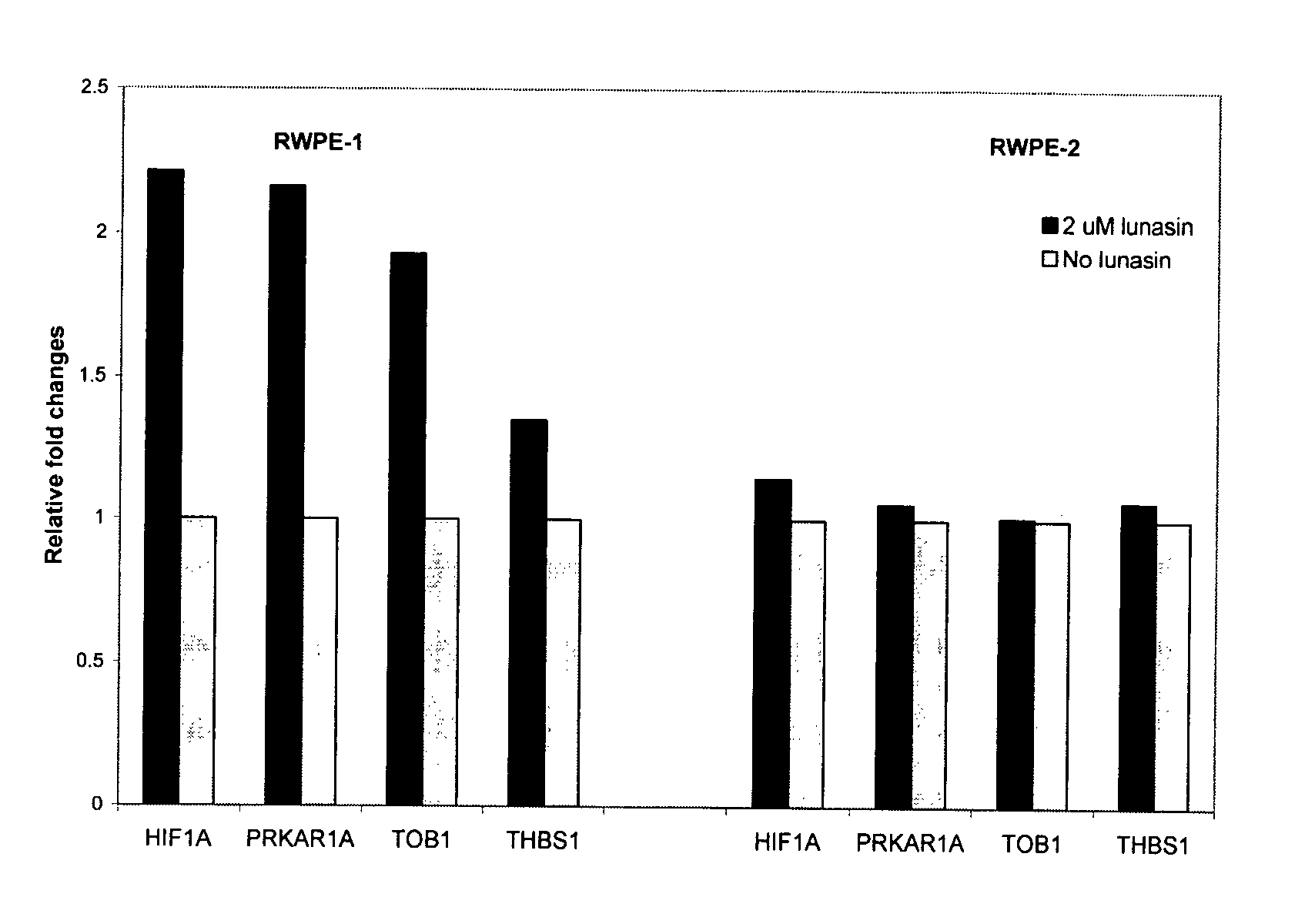
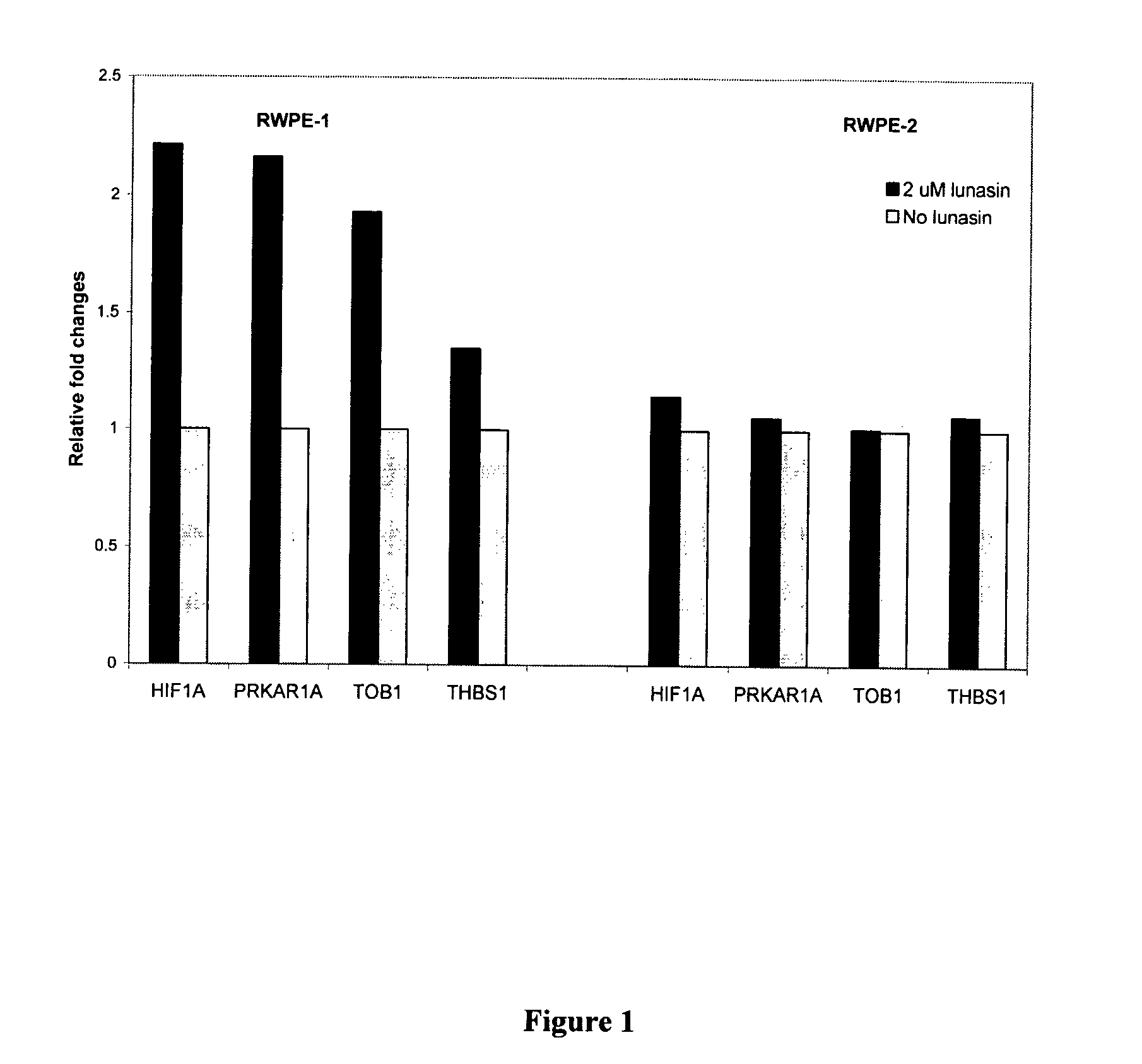
Use of Lunasin Peptide as a Transcriptional Activator to Prevent Cancer and Related Methods for Treatment, Monitoring and Prognosis
Gene expression profiles produced in response to lunasin exposure, wherein such gene expression profiles correlate with anti-neoplastic activity, methods for using such expression profiles for screening potential anti-neoplastic agents, and methods for treatment and monitoring of a subject having a neoplastic disease.
Owner:RGT UNIV OF CALIFORNIA
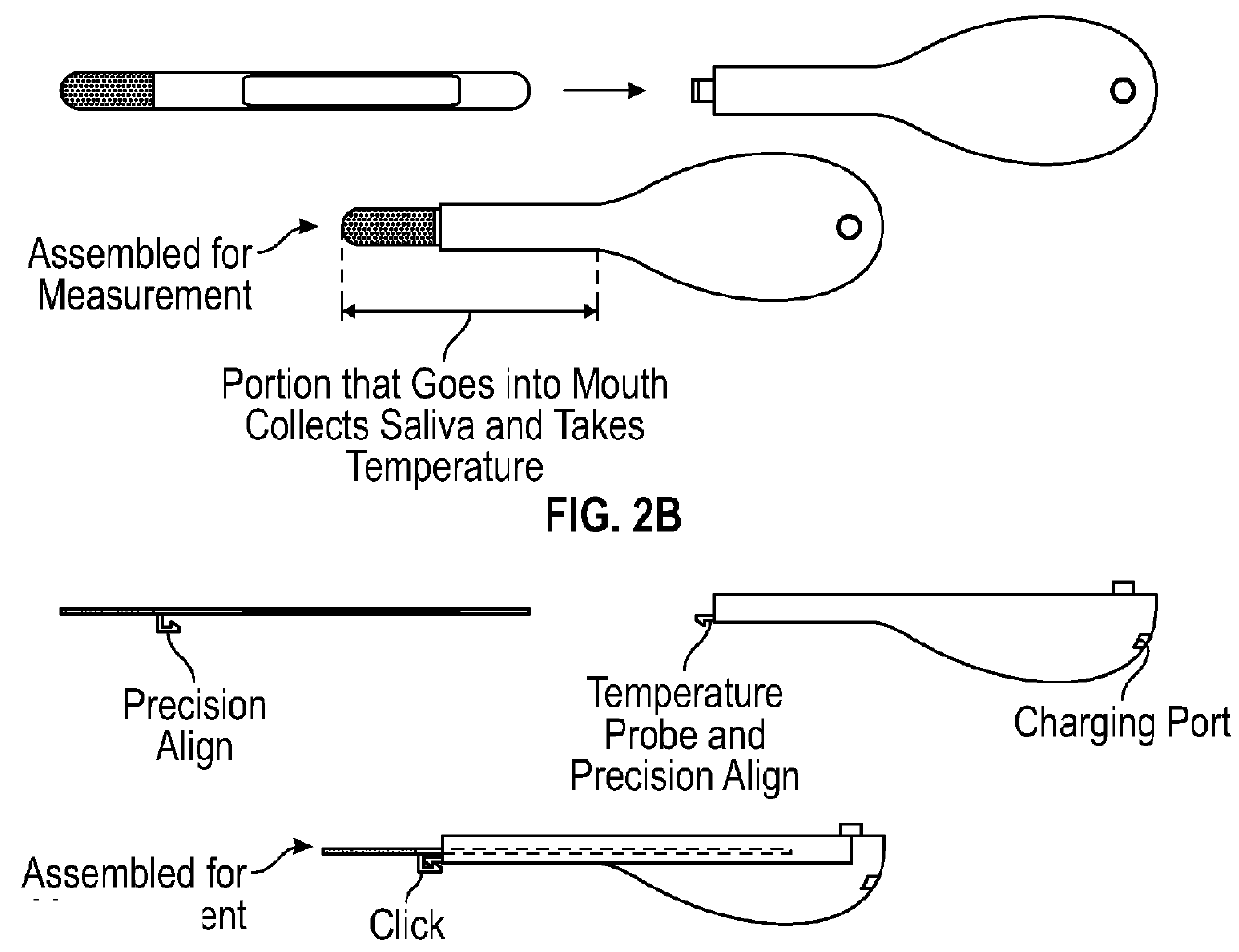
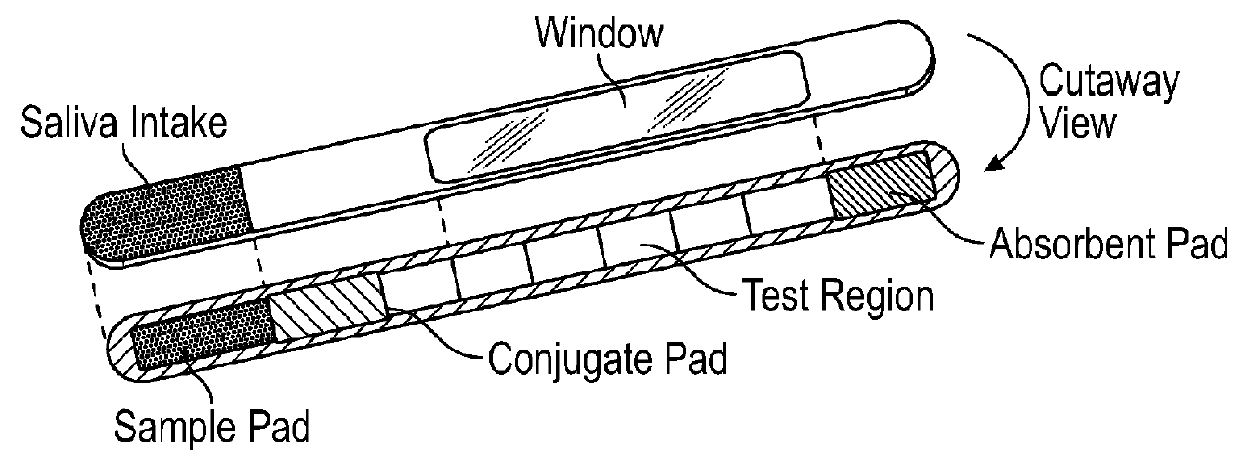
A test device for detecting an analyte in a saliva sample and method of use
InactiveUS20180106799A1Promote solubilizationImprove liquiditySurgeryVaccination/ovulation diagnosticsTherapy monitoringObstetrics
In some aspects, the present disclosure relates to a lateral flow test device, a kit or an instrument comprising the test device, and a method of using the test device, kit, or instrument for quantitatively detecting an analyte in a saliva sample, e.g., a saliva sample from a subject, for example, for assessing hormone(s), ovulation, pregnancy, and / or fertility for a user, e.g., hormonal, ovulation, pregnancy, fertility status, time window, trend, or therapy monitoring or guidance for the user.
Owner:BLUDIAGNOSTICS INC
Atherosclerosis sound power therapeutic system
ActiveCN103893919AEffectiveIn addition to the advantages of effectiveness, it also has the advantages of non-invasiveUltrasound therapyTherapy monitoringCurative effect
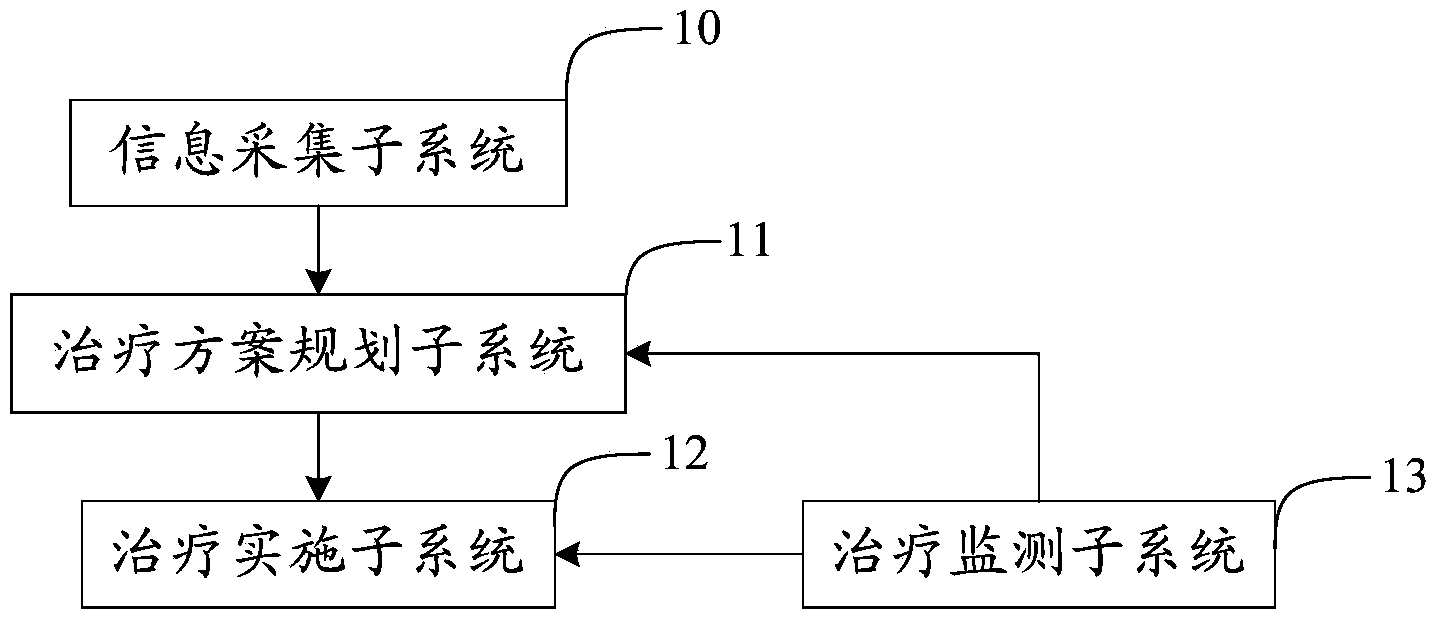
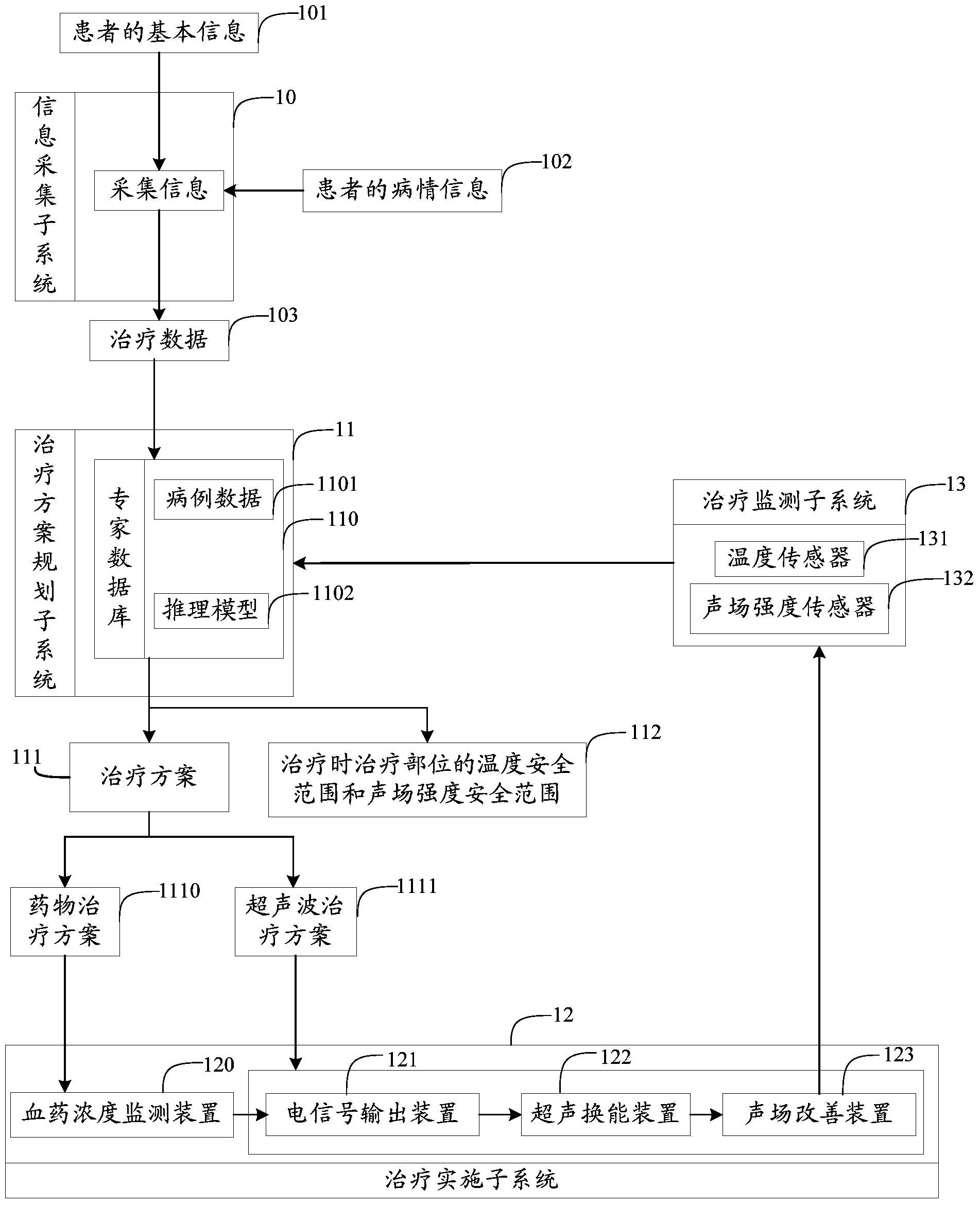
The invention provides an atherosclerosis sound power therapeutic system which comprises an information collection subsystem, a therapeutic schedule planning subsystem, a therapy implementing subsystem and a therapy monitoring subsystem. The information collection subsystem is used for collecting essential information and illness state information of a patient so as to obtain therapeutic data. The therapeutic schedule planning subsystem is used for determining a therapeutic schedule, the temperature safety range of a treated portion in therapy and the sound field intensity safety range of the treated portion in therapy. The therapy implementing subsystem is used for implementing the therapeutic schedule determined by the therapeutic schedule planning subsystem. The therapy monitoring subsystem is used for monitoring the temperature and the sound field intensity of the treated portion in the process of therapy. According to the atherosclerosis sound power therapeutic system, the curative effects that the plaque size can be reduced and plaque stability is improved can be achieved in a short time and the atherosclerosis sound power therapeutic system has the advantages of being noninvasive, convenient to use, capable of conducting therapy repeatedly and the like which are not available for an intervention method.
Owner:哈尔滨声诺医疗科技有限公司
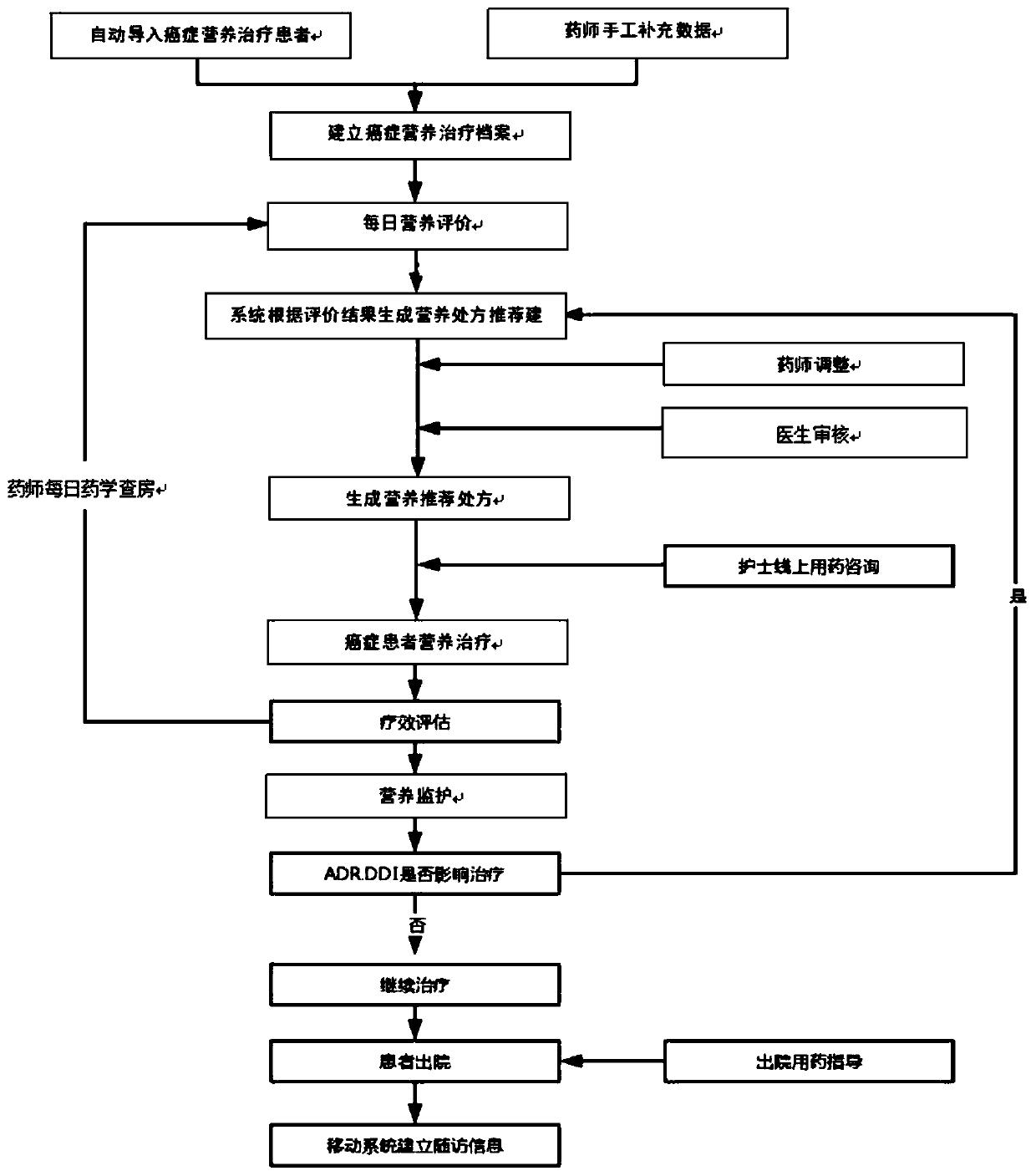
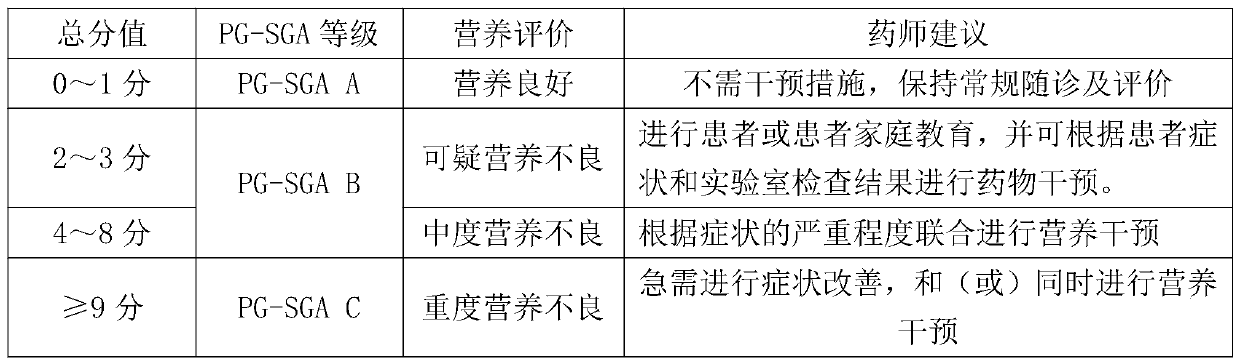
Cancer patient nutrition management system
InactiveCN111445981ATechnical Content StandardizationHigh repeatability of quality controlHealth-index calculationNutrition controlTherapy monitoringPharmacy medicine
The invention provides a cancer patient nutrition management system, and relates to the technical field of cancer nutrition therapies. The system comprises a patient basic information module, a grouping information module, a nutrition evaluation module, a medicine therapy monitoring module, a patient follow-up visit module and a therapy management log module. The system automatically marks patients in a grading mode, and rapid grouping is achieved; a pharmacist carries out nutrition evaluation on the patient every day, and an improved PG-SGA method is adopted for subjective overall evaluationof the patient; and the system records comprehensively-generated nutrition prescription recommendation suggestions according to nutrition evaluation, the nutrition prescription recommendation suggestions are adjusted by pharmacists and then pushed to doctors for checking, and nutrition recommendation prescriptions are obtained after checking of the doctors. The system provided by the invention cansimultaneously realize the functions of automatic grading marking of cancer nutrition treatment patients, online nutrition evaluation, linked checking of nutrition prescriptions by doctors and pharmacists, online communication, regular follow-up visit tables, past treatment file storage and retrieval and the like, saves a lot of time for medical workers, and improves the working efficiency.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Caspase-3 Substrate Comprising Imaging Agents
InactiveUS20080286201A1Fast imagingBig gapHydrolasesTetrapeptide ingredientsTherapy monitoringDisease
Owner:GUILBERT BENEDICTE +5
Chronic insomnia remote cognitive-behavioural therapy system
InactiveCN104644128ARealize personalized remote non-drug treatment planShorten the timeDiagnostic recording/measuringSensorsTherapy monitoringThe Internet
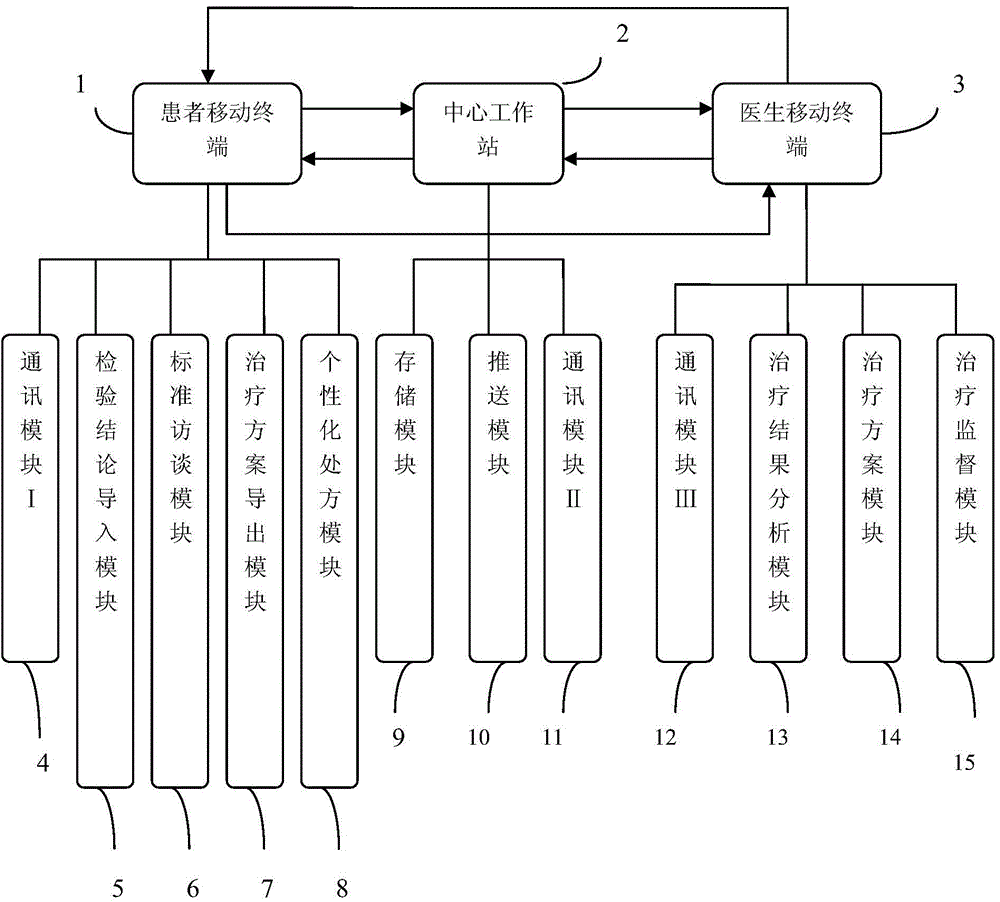
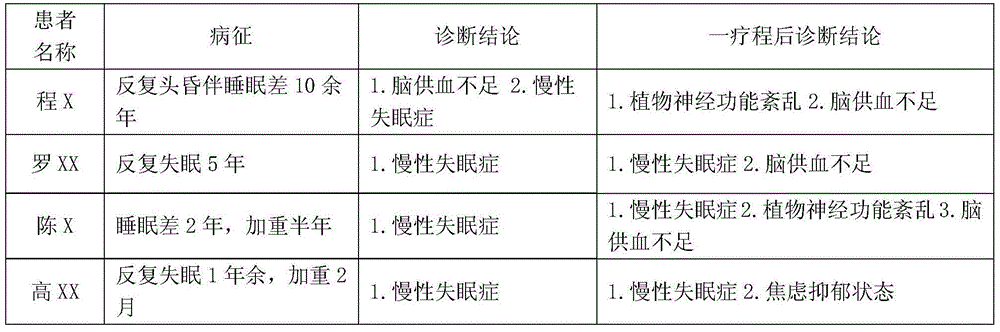
The invention discloses a chronic insomnia (CI) remote cognitive-behavioural therapy system. The system comprises at least one patient mobile terminal; the patient mobile terminal is connected with a central workstation by internet; the central workstation is connected with at least one doctor mobile terminal by the internet. The patient mobile terminal comprises a patient mobile communication platform comprising a communication module I, an inspection conclusion import module, a standard interview module, a therapeutic schedule export module and an individualized prescription module; the central workstation comprises a server and a personal computer; the server comprises an optimizing module, a communication module II and a storage module; the doctor mobile terminal comprises a communication module III, a therapeutic schedule module, a therapy monitoring module and a therapy result analysis module. The cognitive-behavioural therapy basic interview is standardized by using the internet technology, the long-distance persistent CI cognitive-behavioural therapy is realized, and more CI patients are treated under limited medical resources.
Owner:中国人民解放军陆军特色医学中心 +1
Biomarkers of chronic pelvic pain syndrome
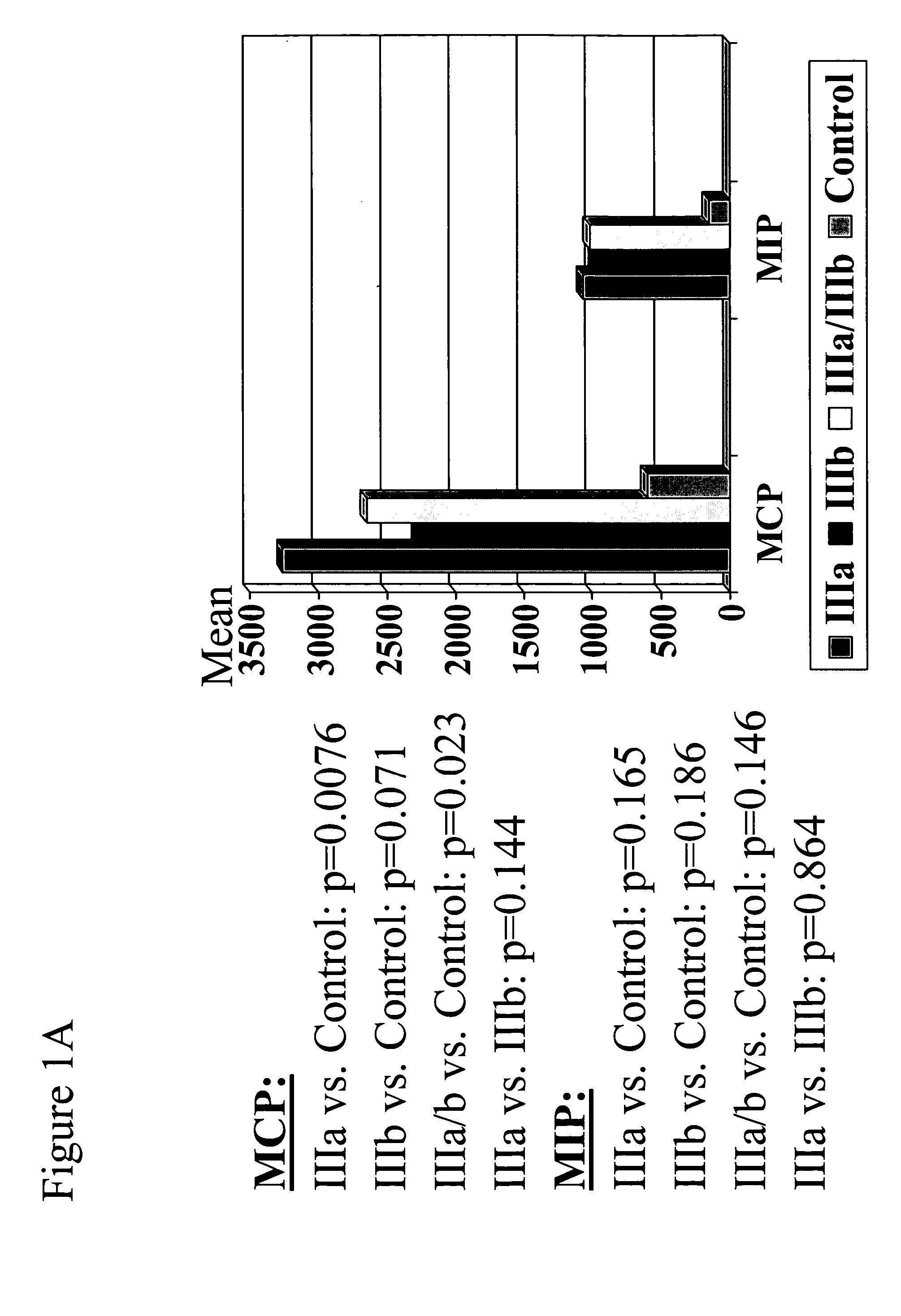
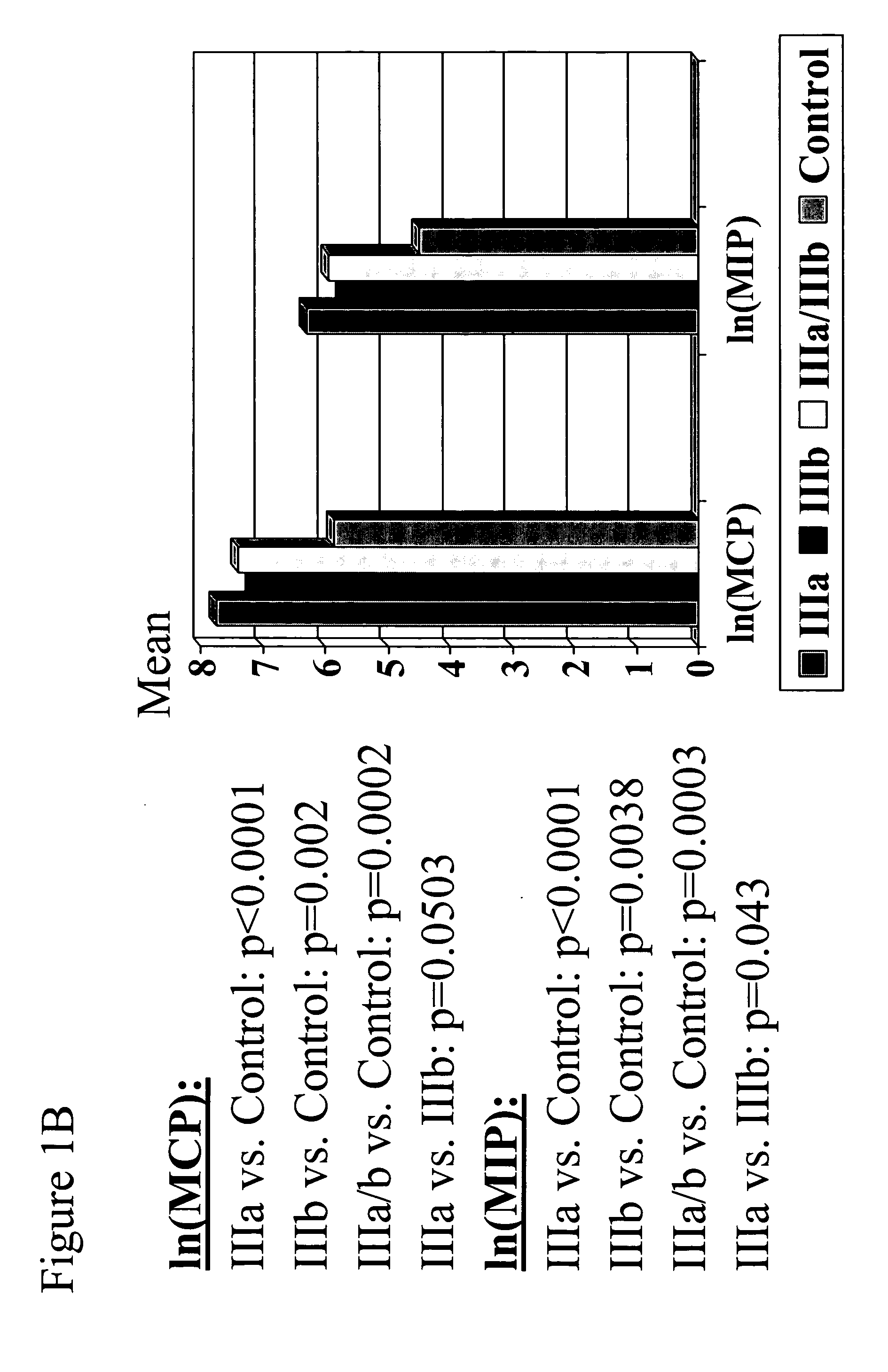
ActiveUS20050277140A1Reduce the amount requiredMicrobiological testing/measurementDisease diagnosisTherapy monitoringMedicine
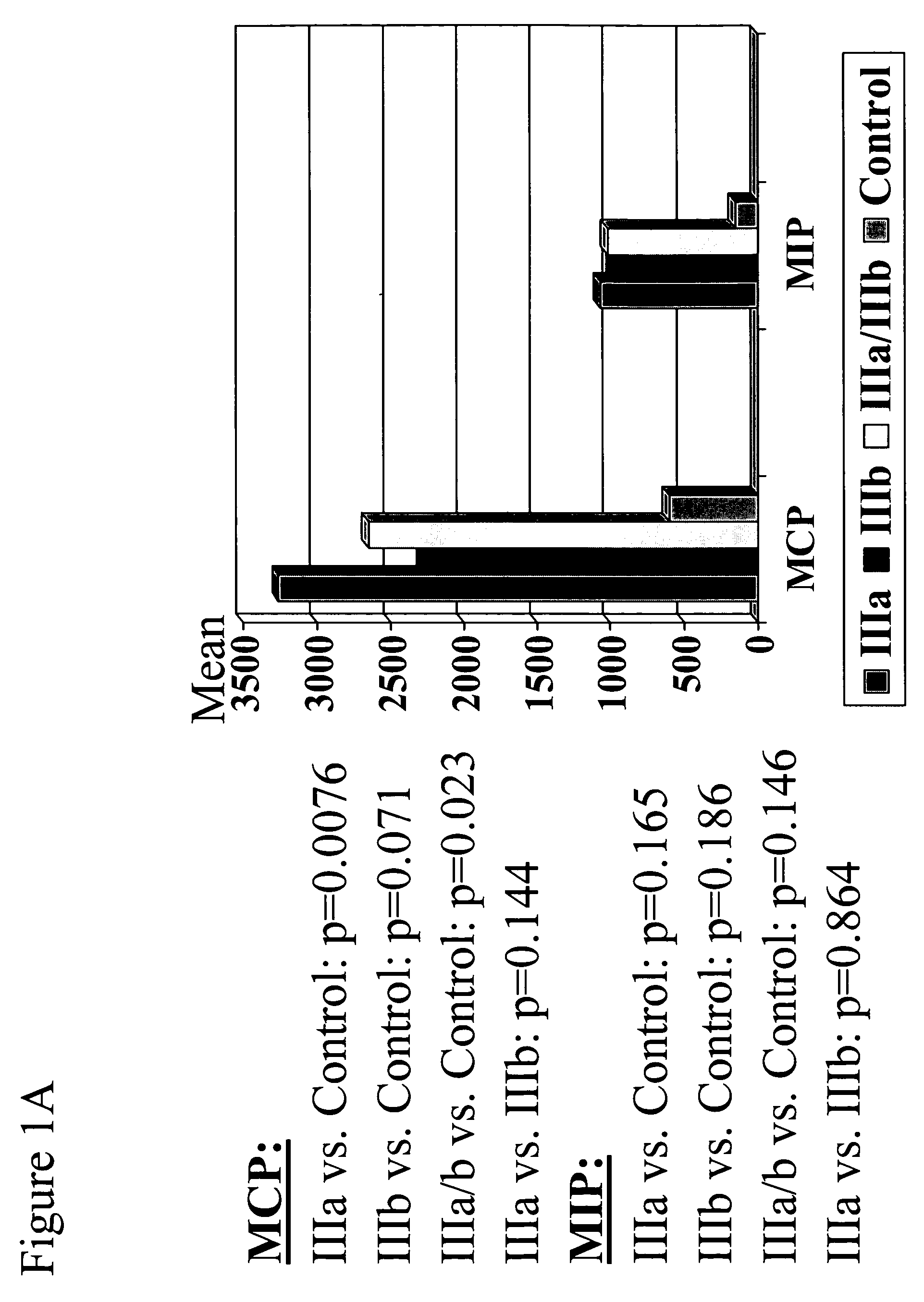
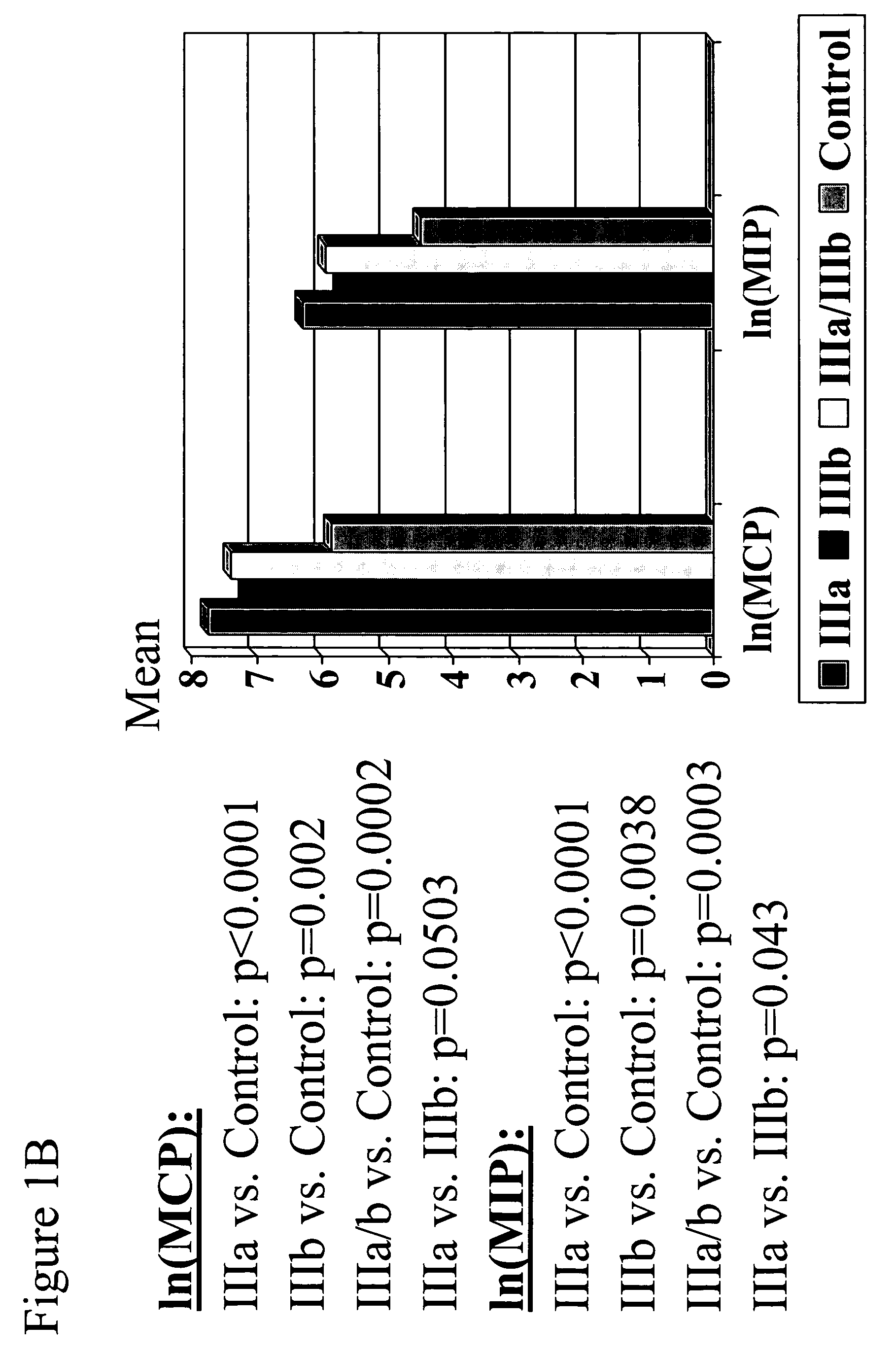
The present invention provides biomarkers of chronic pelvic pain syndrome for use in diagnosis, drug screening, therapy monitoring, research and therapeutic applications. In particular, the present invention provides MCP-1 and MIP-1α as biomarkers of chronic pelvic pain syndrome.
Owner:NORTHWESTERN UNIV
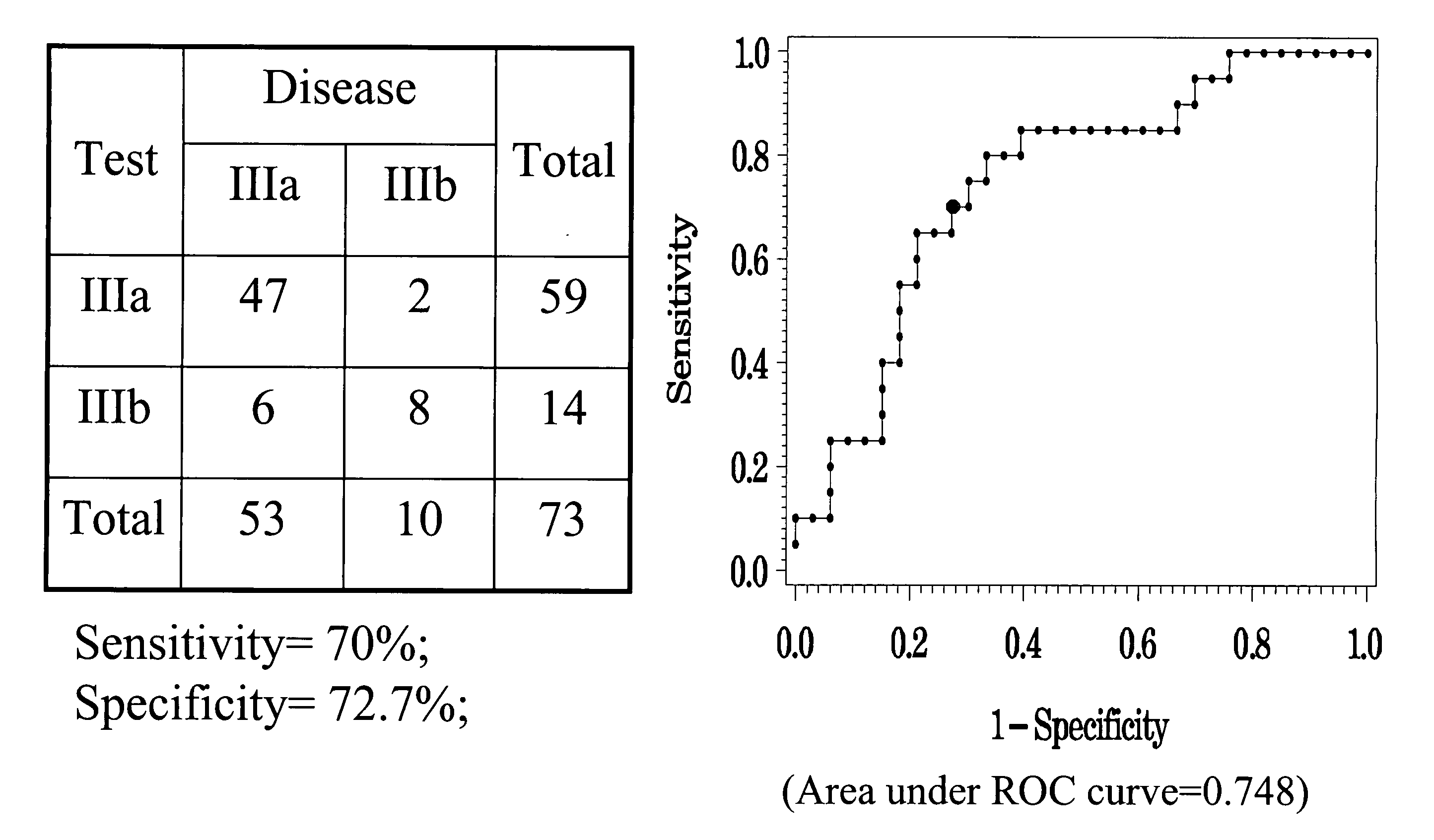
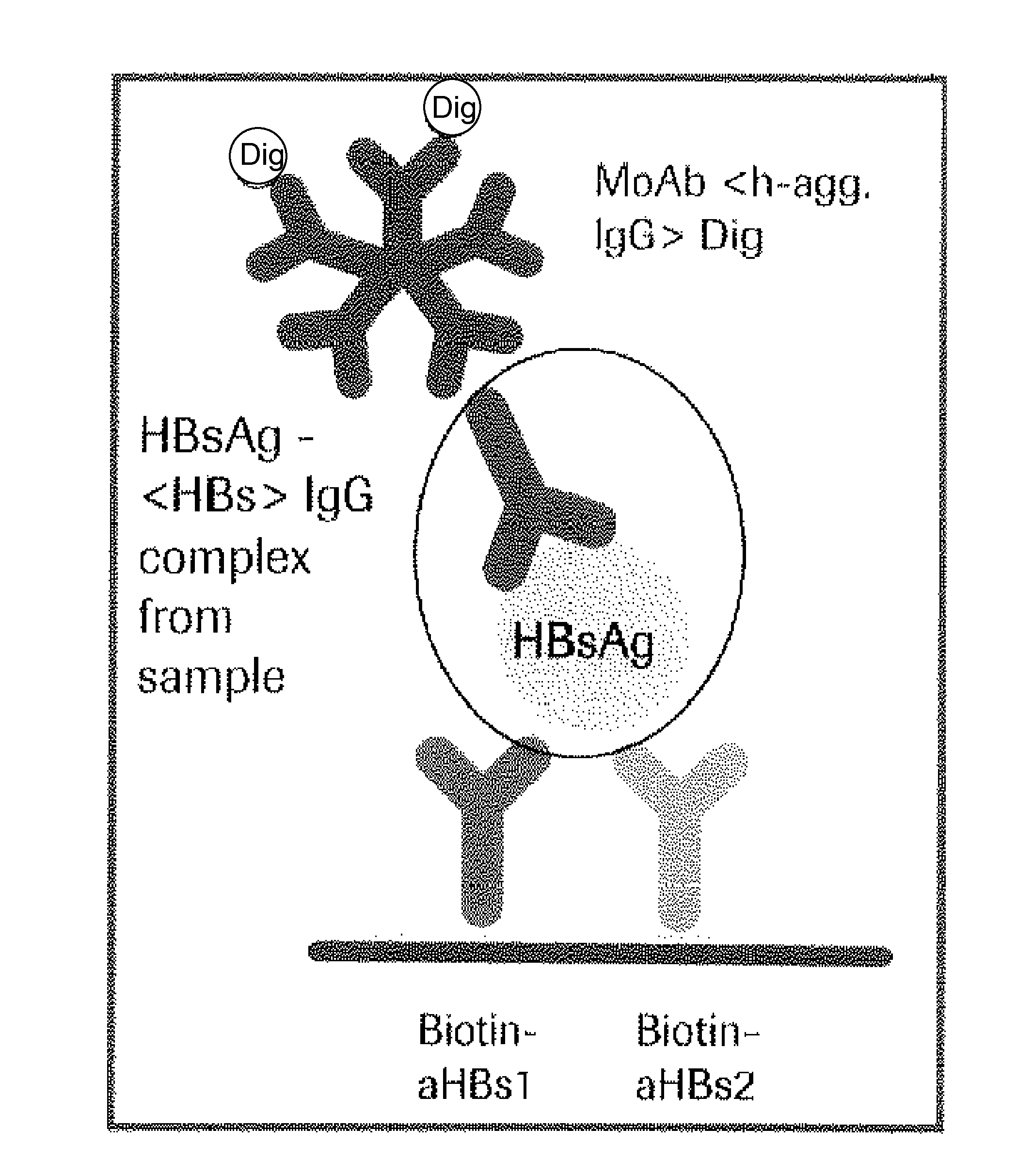
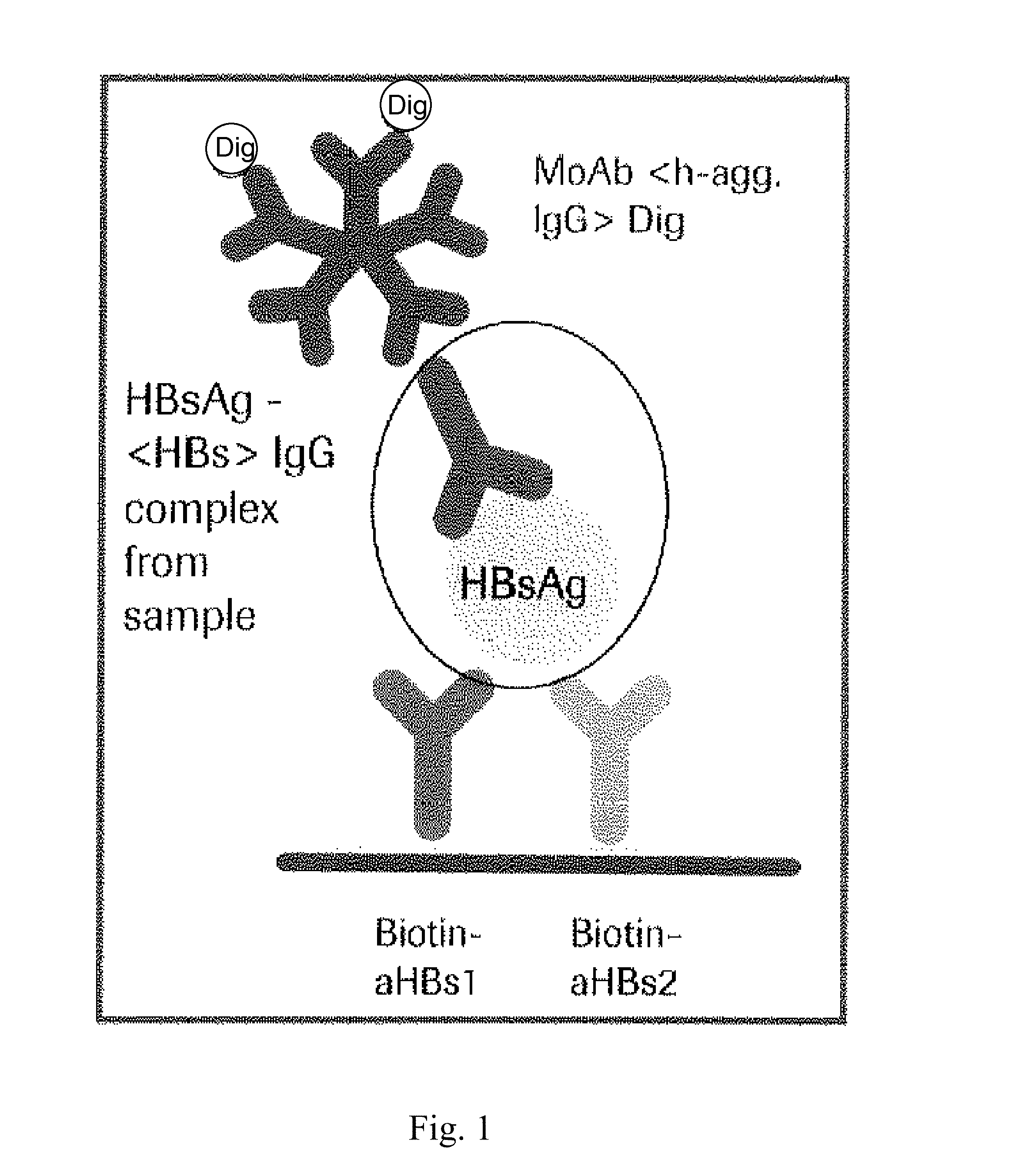
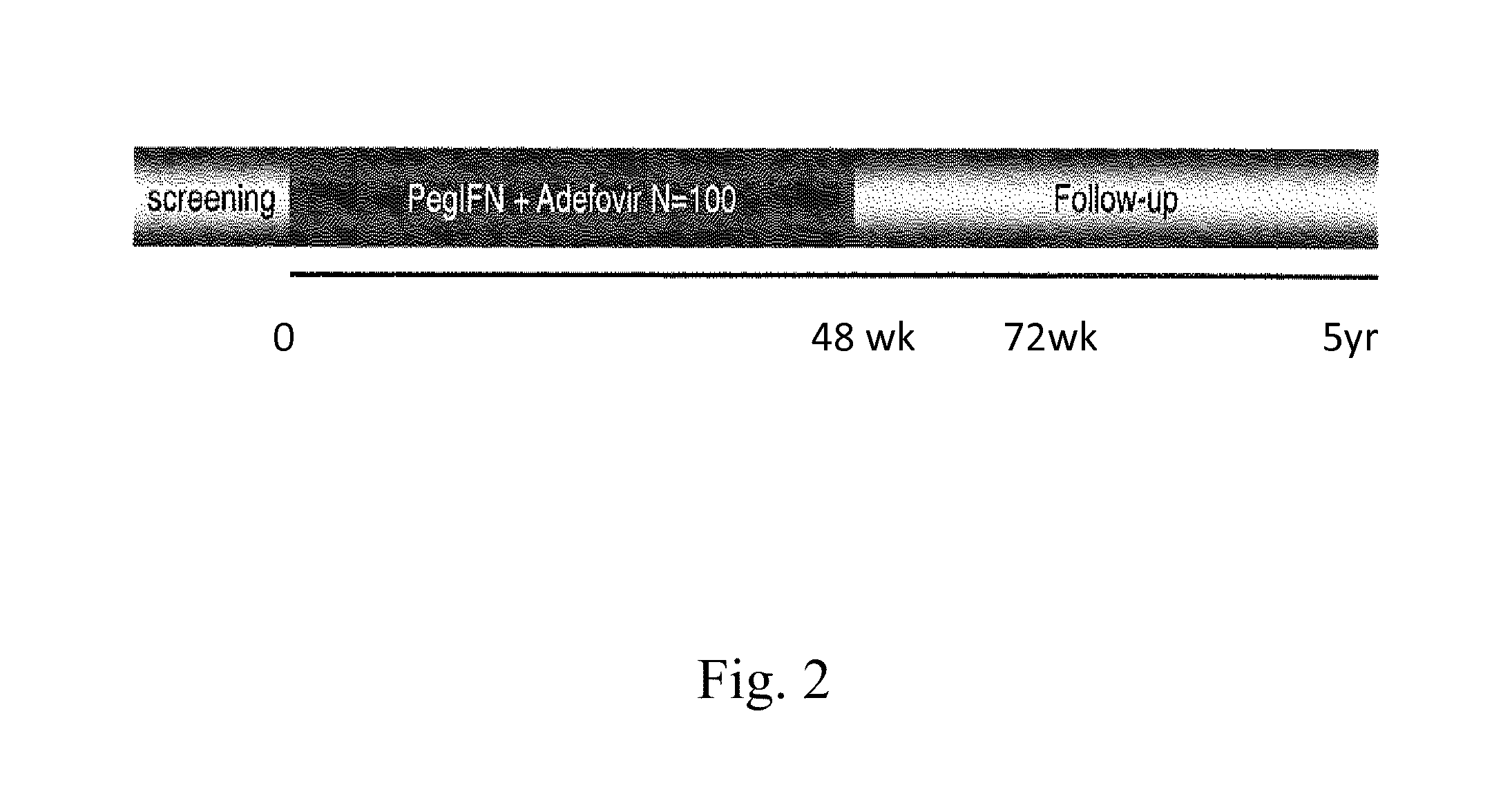
Hbv immunocomplexes for response prediction and therapy monitoring of chronic hbv patients
InactiveUS20150192583A1Bioreactor/fermenter combinationsBiological substance pretreatmentsInterferon therapyTherapy monitoring
The present invention relates to a method for identifying a subject suffering from hepatitis B virus (HBV) infection as being susceptible to interferon treatment, said method comprising the steps of a) determining, in a sample of said subject, the amount of HBV immune complexes, b) comparing the amount of HBV immune complexes obtained in step a) to a reference value, and c) identifying a subject suffering from HBV infection as being susceptible to interferon treatment based on the result of the comparison made in step b). The present invention further relates to the use of the determination of the amount of HBV immune complexes in a sample from a subject suffering from HBV infection and of a detection agent for HBV immune complexes for identifying a subject suffering from HBV infection as being susceptible to interferon treatment. Furthermore, the present invention relates to a device and a kit allowing identifying a subject suffering from HBV infection as being susceptible to interferon treatment.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC +1
Caspase-3 substrate comprising imaging agents
InactiveCN101155602ATetrapeptide ingredientsRadioactive preparation carriersDiseaseTherapy monitoring
The present invention relates to diagnostic imaging agents for in vivo imaging. The imaging agents comprise a synthetic caspase-3 substrate peptide labelled with an imaging moiety suitable for diagnostic imaging in vivo. The invention also provides radiopharmaceutical compositions comprising the imaging agents, together with kits for the preparation of the radiopharmaceuticals. Also described are non-radioactive precursors suitable for the preparation of the imaging agents. The imaging agents are useful for the diagnostic imaging and or therapy monitoring in vivo of various disease states where caspase-3 is involved.
Owner:GE HEALTHCARE LTD
Imaging neurological disease
ActiveUS20160183897A1Radioactive preparation carriersMedical automated diagnosisDiseaseTherapy monitoring
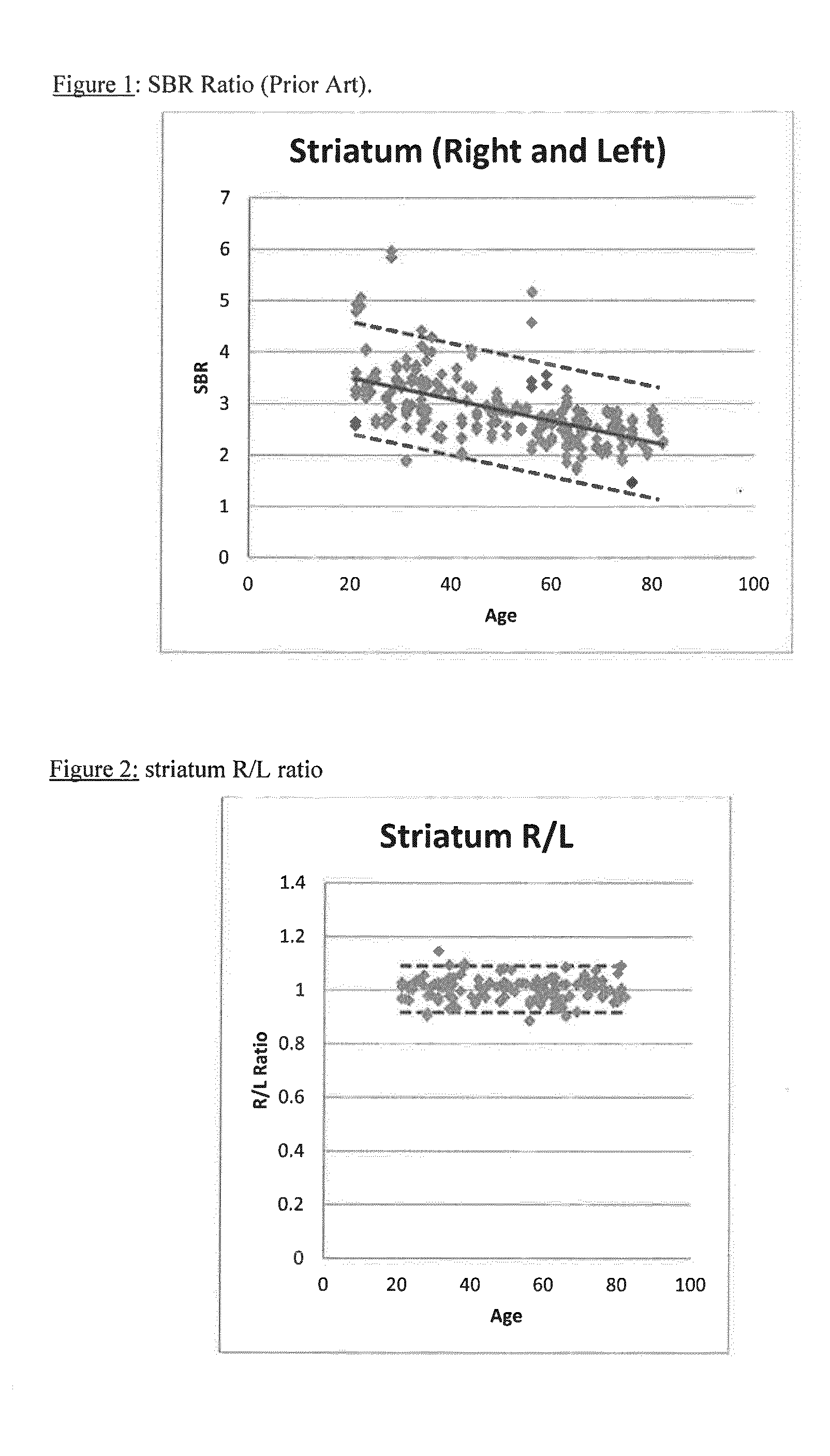
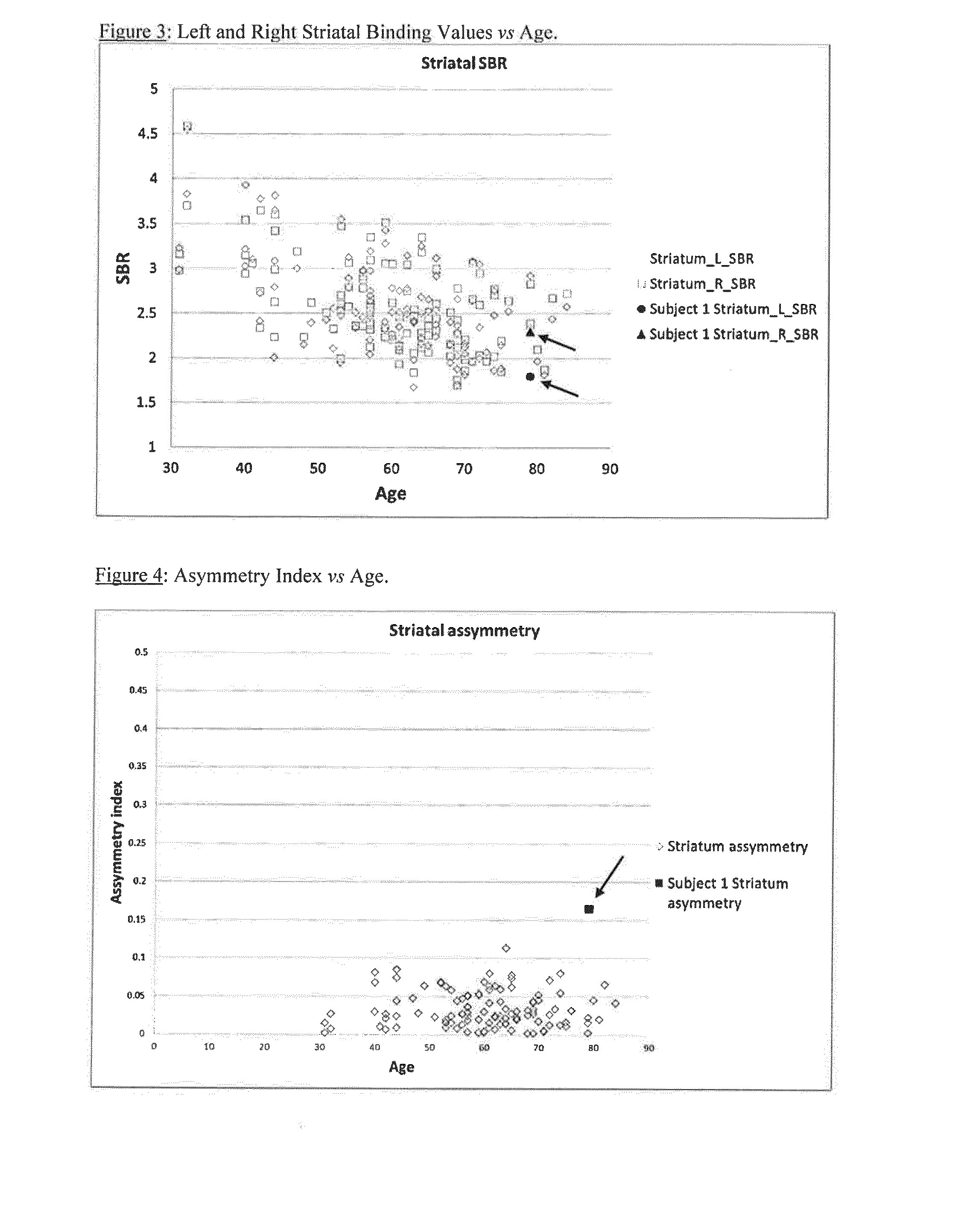
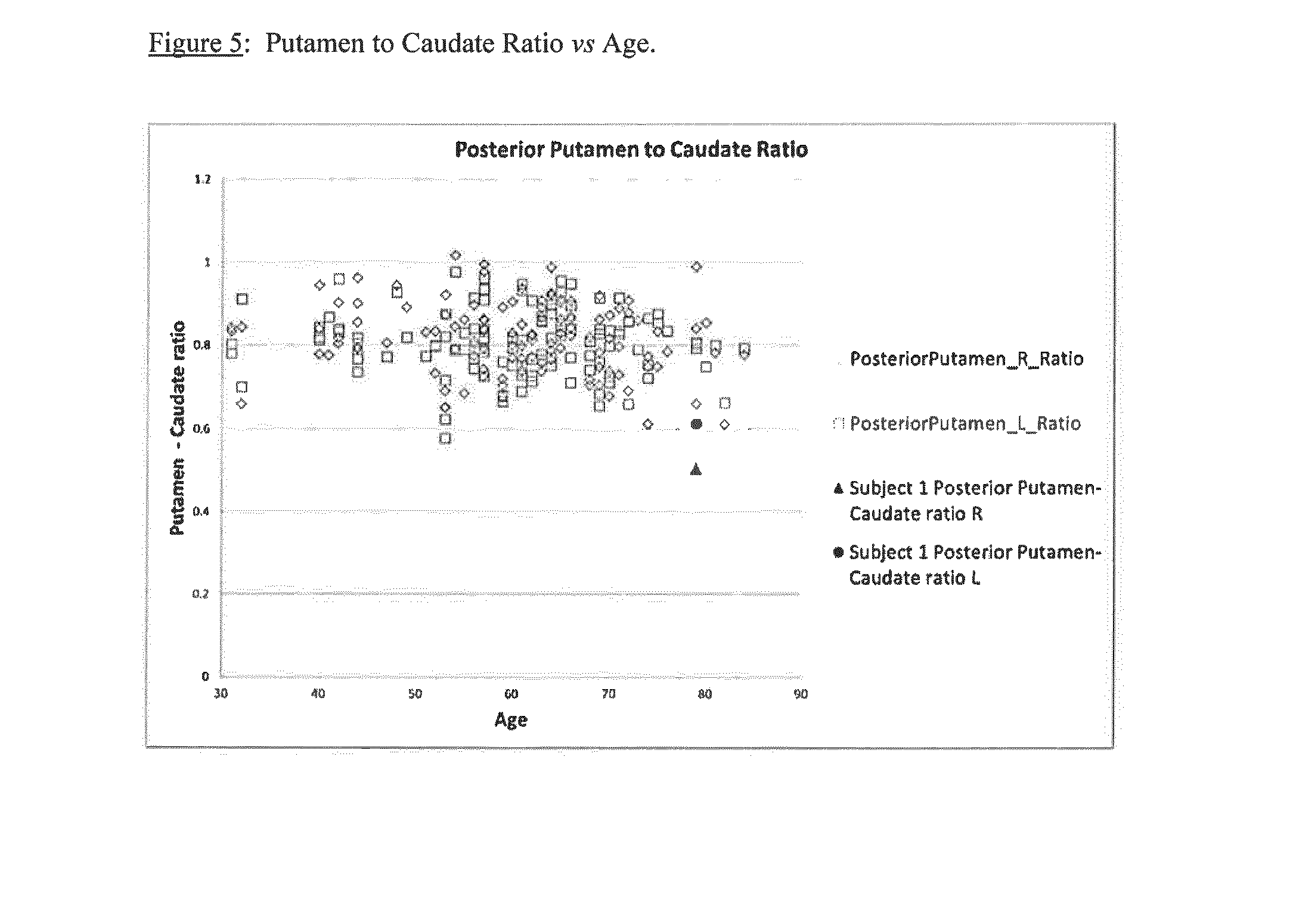
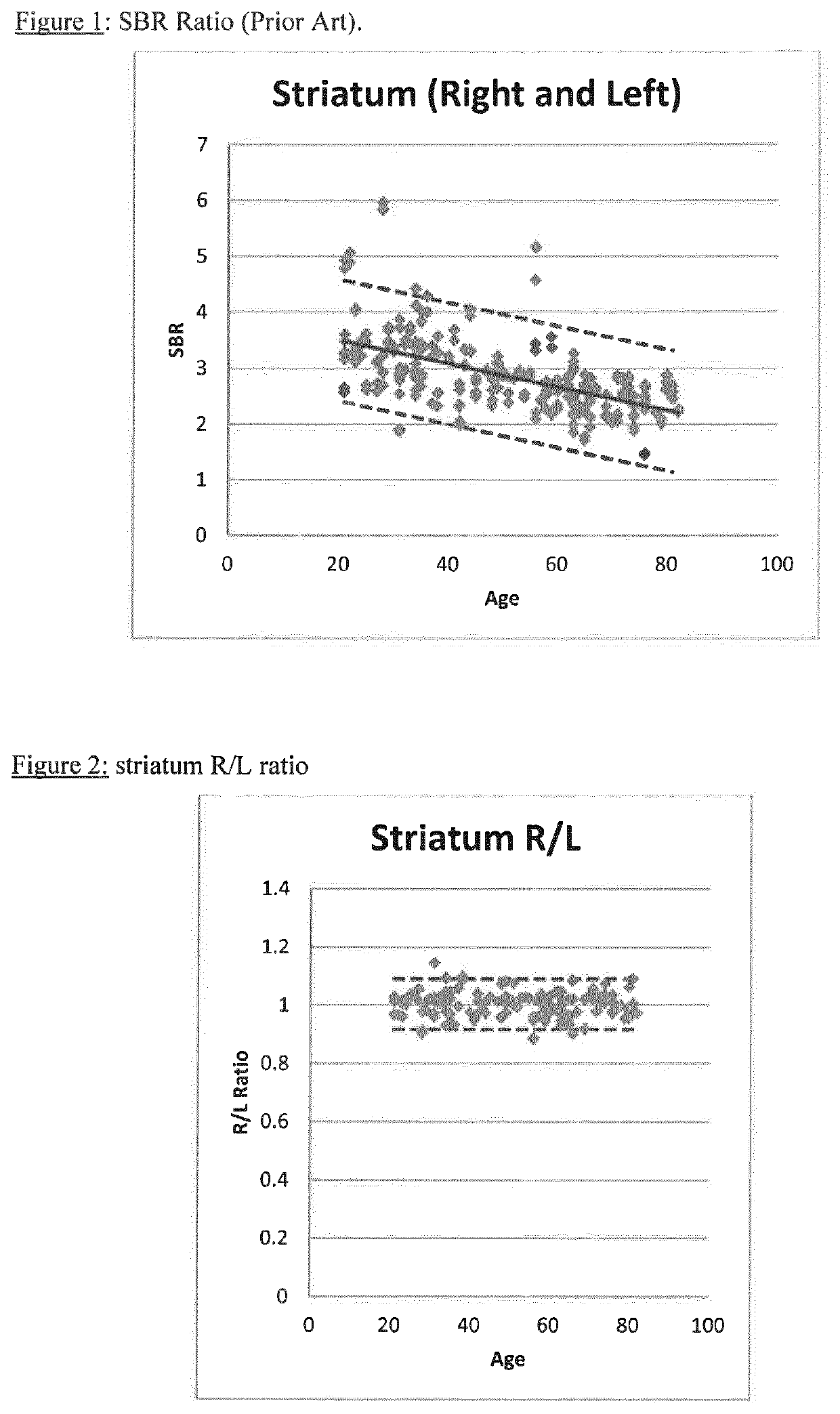
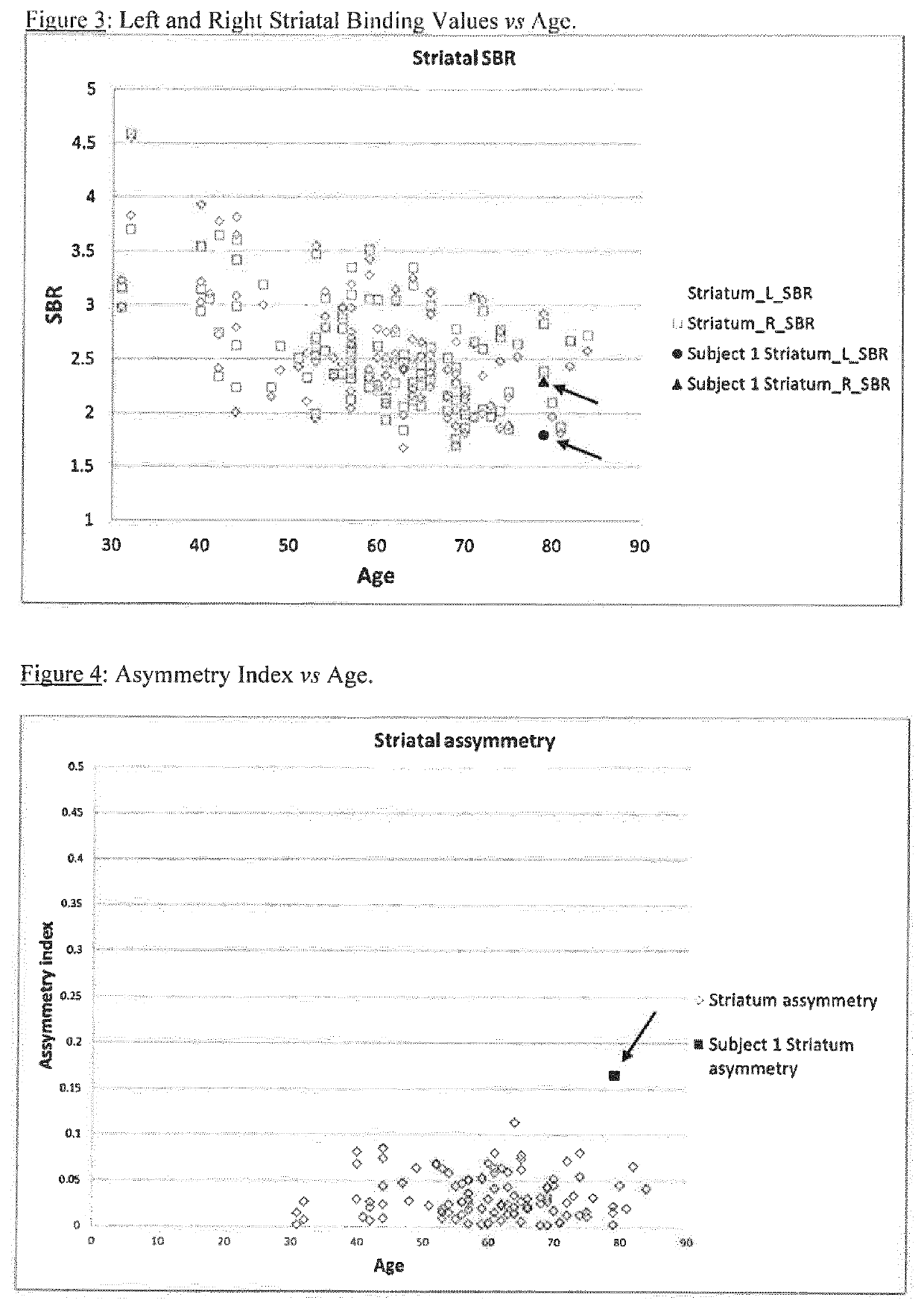
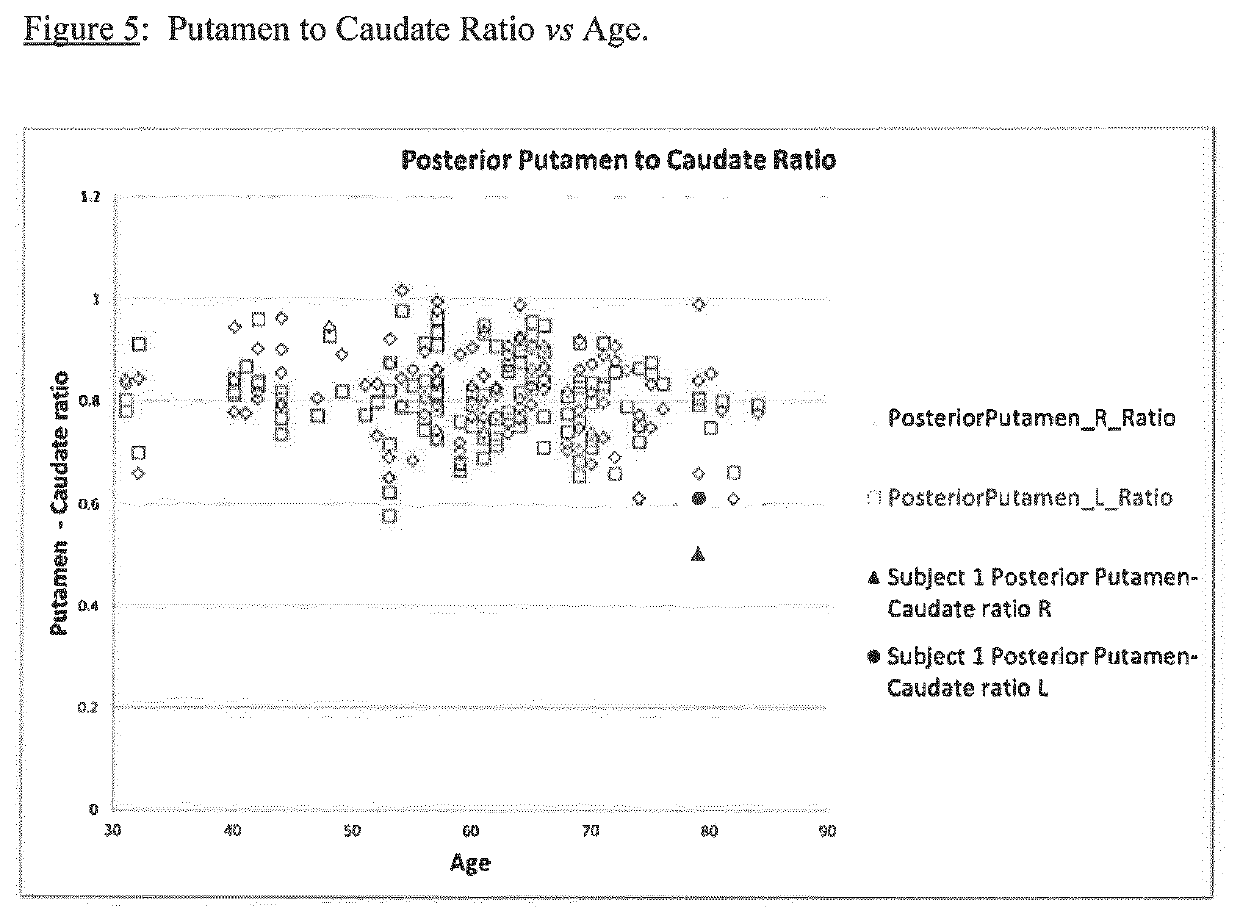
A method of imaging to permit calculation of left:right striatum uptake ratios is provided, and the degree of asymmetry used to assist in the diagnosis of neurological diseases. Also provided are a method of diagnosis, method of patient selection, and method of therapy monitoring using the imaging method, and software tools for use in the method.
Owner:GE HEALTHCARE LTD
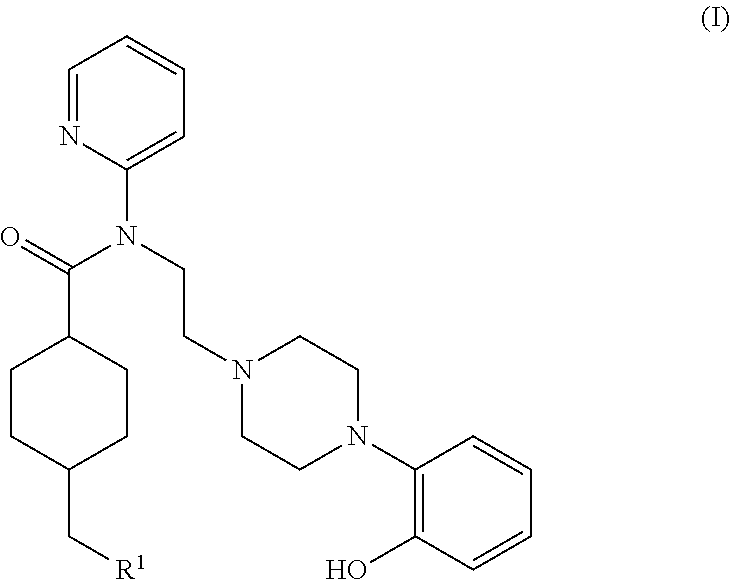
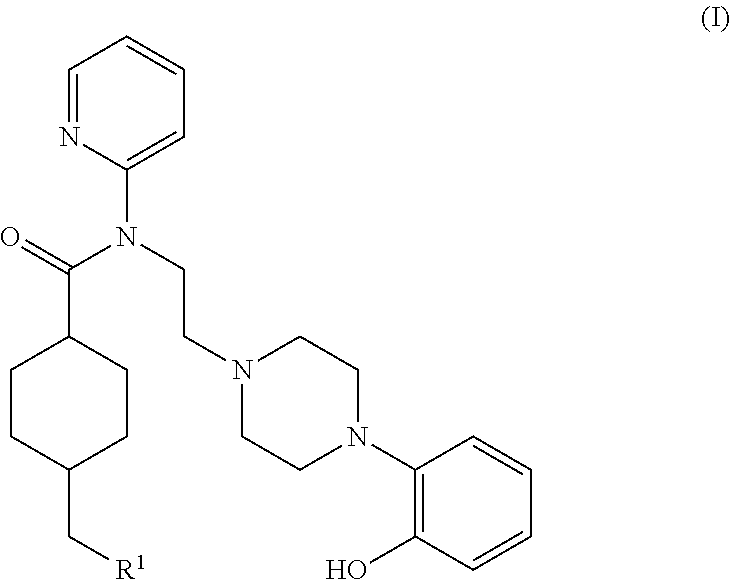
5ht1a antagonist useful for in vivo imaging
InactiveUS20140140928A1Beneficial pharmacological profileReadily radiolabeledNervous disorderOrganic chemistryTherapy monitoringMedicine
The present invention provides a novel compound of formula (I) useful for in vivo imaging of 5-HT1 A receptors in a subject. Also provided by the present invention is a precursor compound useful in the preparation of the compound of the invention, as well as said method of preparation. The present invention additionally provides methods for the use of the compound of the invention in an in vivo imaging method, and use of that in vivo imaging method in diagnosis and therapy monitoring.
Owner:GE HEALTHCARE LTD
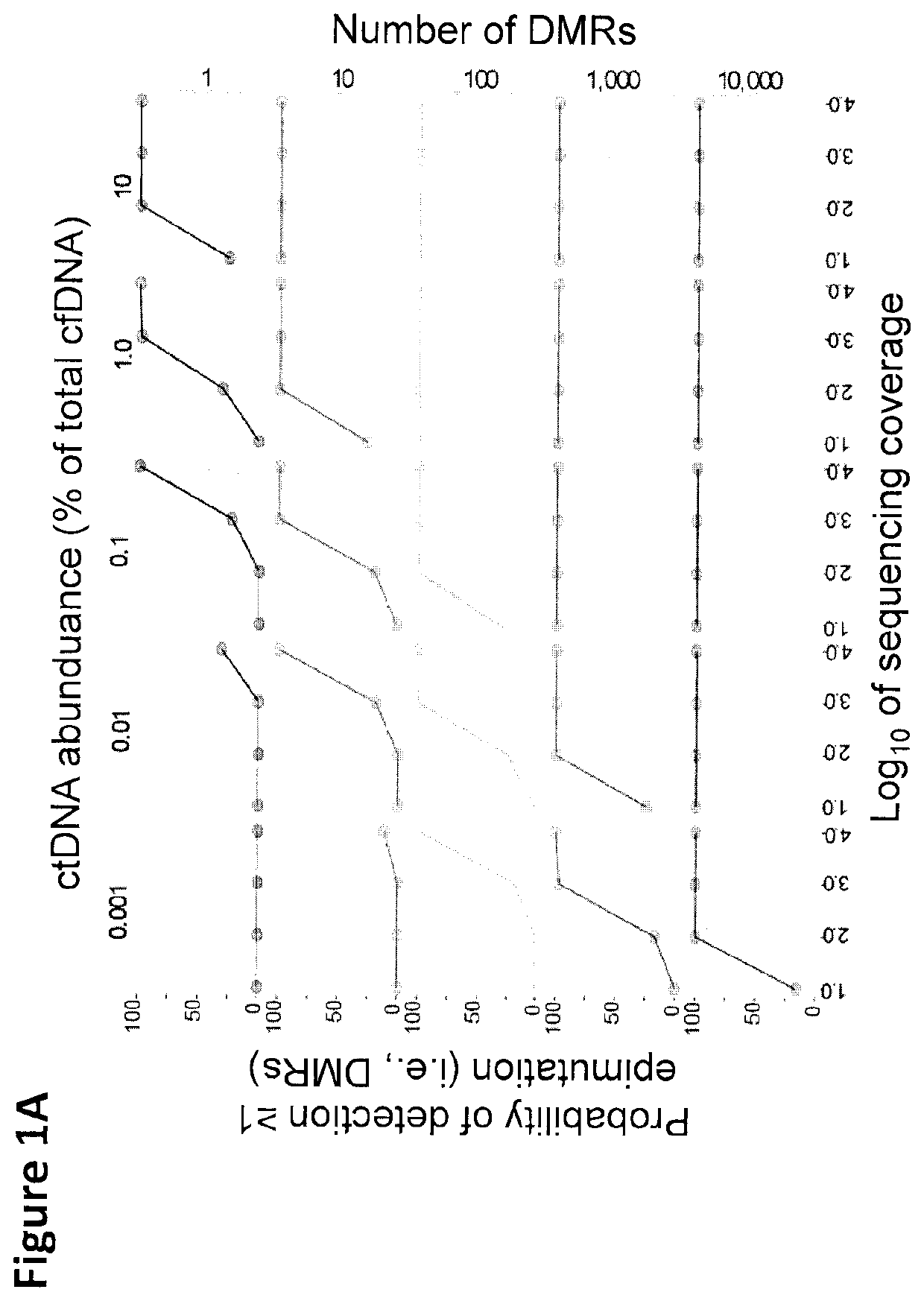
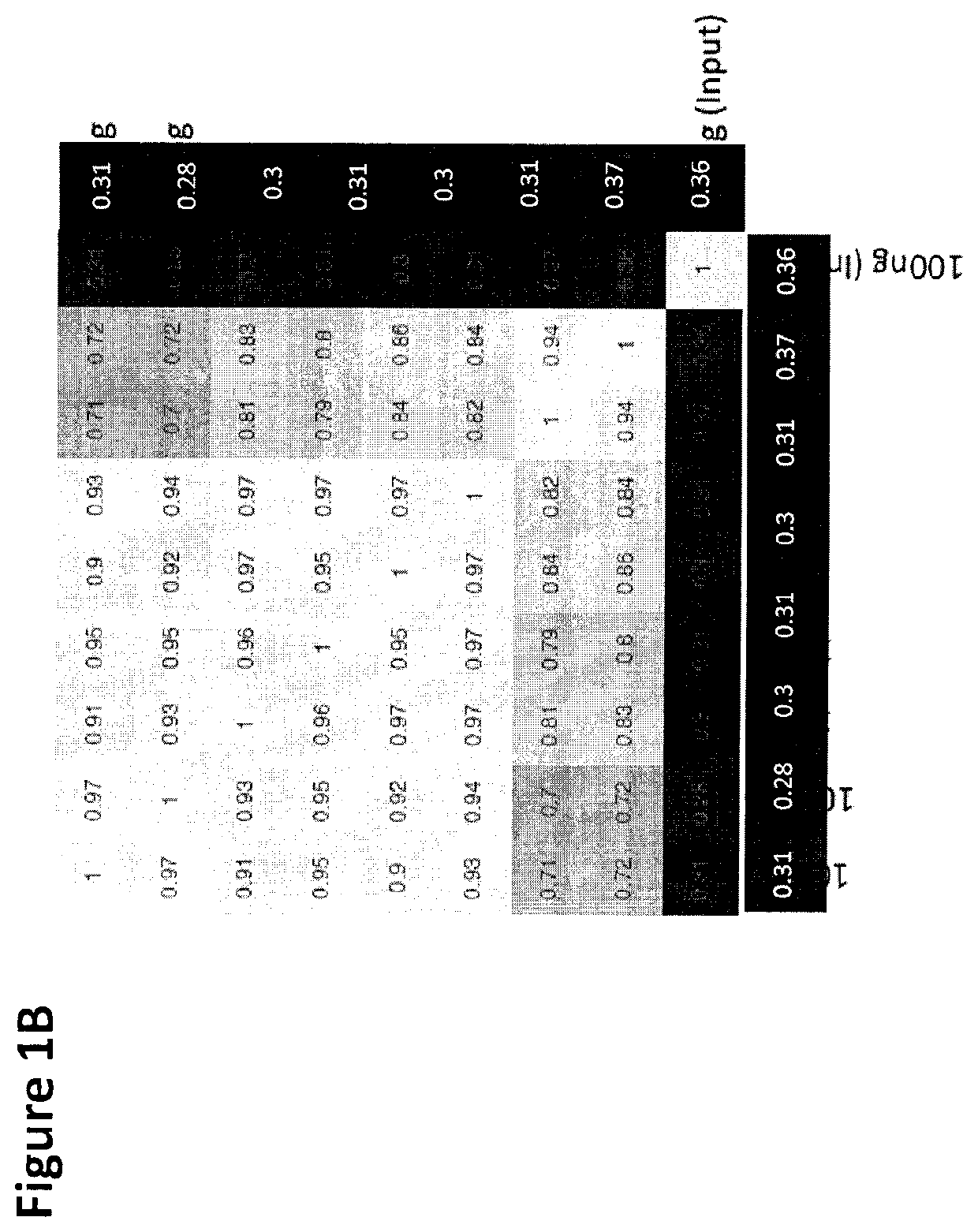
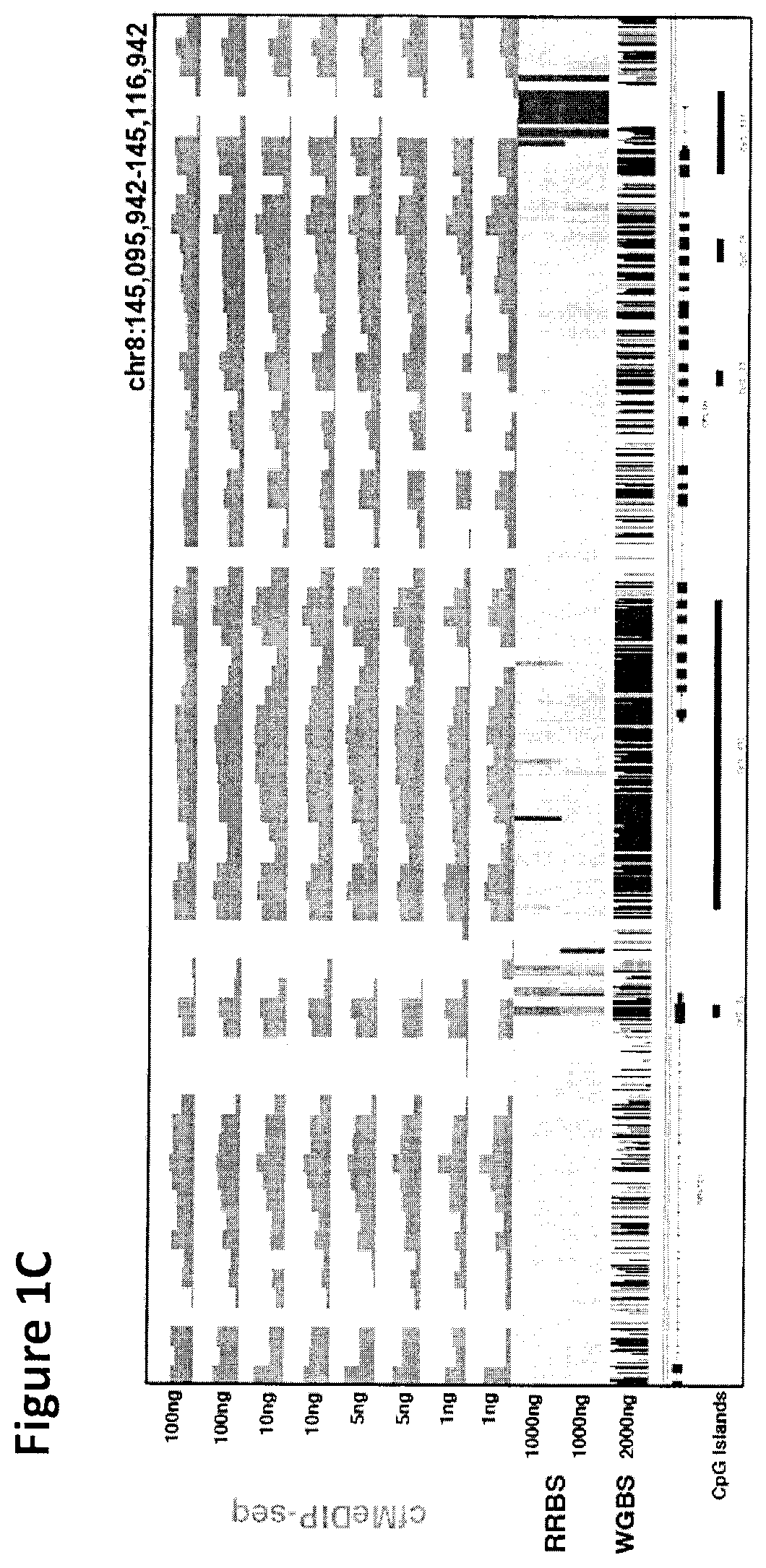
Cancer detection, classification, prognostication, therapy prediction and therapy monitoring using methylome analysis
There is described herein a method of detecting the presence of DNA from cancer cells in a subject comprising: providing a sample of cell-free DNA from a subject; subjecting the sample to library preparation to permit subsequent sequencing of the cell-free methylated DNA; optionally denaturing the sample; capturing cell-free methylated DNA using a binder selective for methylated polynucleotides; sequencing the captured cell-free methylated DNA; comparing the sequences of the captured cell-free methylated DNA to control cell-free methylated DNAs sequences from healthy and cancerous individuals; identifying the presence of DNA from cancer cells if there is a statistically significant similarity between one or more sequences of the captured cell-free methylated DNA and cell-free methylated DNAs sequences from cancerous individuals.
Owner:UNIV HEALTH NETWORK +1
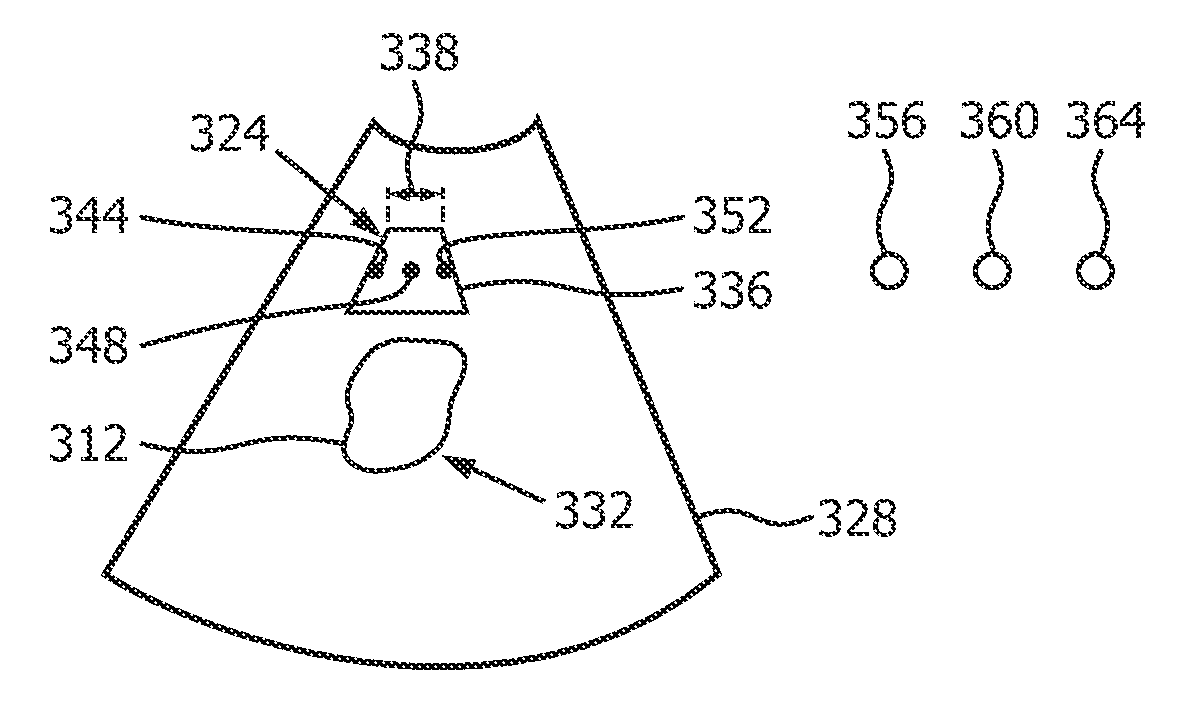
Ultrasound shear wave elastography featuring therapy monitoring
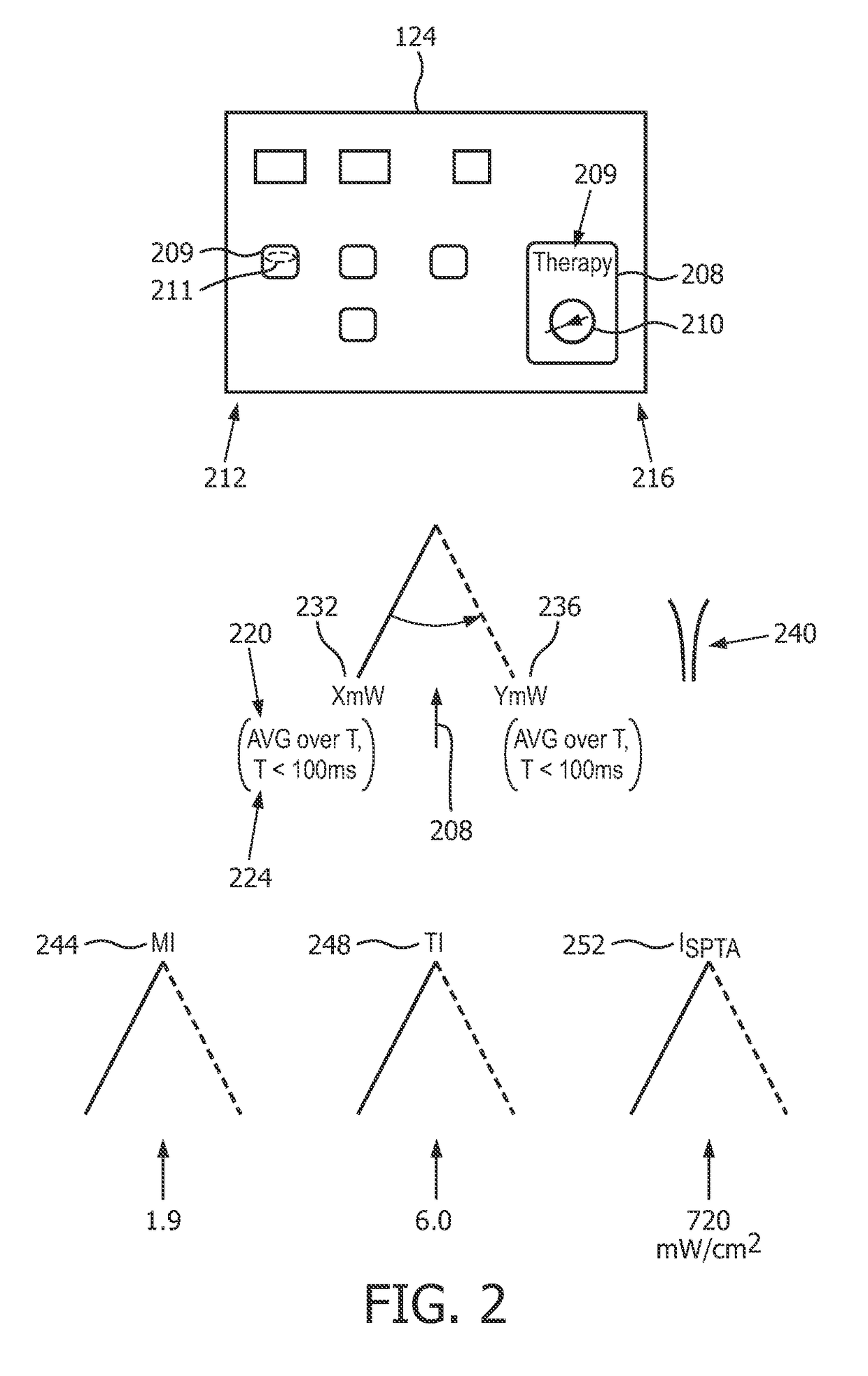
ActiveUS20170188997A1Robust measurementExcellent image reproducibilityOrgan movement/changes detectionControlling energy of instrumentTherapy monitoringSonification
In a diagnostic scanner for shear wave elastography imaging an ultrasound exposure safety processoris is configured for spatially relating respective definitions of an imaging zone (324), and an extended dead-tissue zone (312) that includes both a dead-tissue zone and a surrounding margin. Based on whether a push pulse focus (344, 348, 352) is to be within the extended dead-tissue zone, the processor automatically decides as to a level of acoustic power with which the pulse is to be produced. If it is to be within, the pulse may be produced with a mechanical index, a thermal index, and / or a spatial-peak-temporal-average intensity that exceeds respectively 1.9, 6.0 and 720 milliwatts per square centimeter. The imaging zone may be definable interactively so as to dynamically trigger the deciding and the producing, with optimal push pulse settings being dynamically derived automatically, without the need for user intervention. A display of multiple push pulse sites (344-352) allows user manipulation of spatial definition indicia (336) to dynamically control displacement tracking.
Owner:KONINKLJIJKE PHILIPS NV
Identifying increased susceptibility to schizophrenia
InactiveUS8048622B2Microbiological testing/measurementBiological testingTherapy monitoringBipolar mood disorder
The present invention provides diagnostic markers of neuropsychiatric disorders (e.g., schizophrenia, schizoaffective disorder or serious mood disorders including bipolar disorder and recurrent unipolar disorder) for use in diagnosis, drug screening, therapy monitoring, research and therapeutic applications. In particular, the present invention provides SLC18A1 and TAAR2, and mutations therein, as biomarkers of neuropsychiatric disorders.
Owner:RGT UNIV OF MICHIGAN
Imaging neurological disease
ActiveUS11051777B2Radioactive preparation carriersMedical automated diagnosisTherapy monitoringNervous system
A method of imaging to permit calculation of left:right striatum uptake ratios is provided, and the degree of asymmetry used to assist in the diagnosis of neurological diseases. Also provided are a method of diagnosis, method of patient selection, and method of therapy monitoring using the imaging method, and software tools for use in the method.
Owner:GE HEALTHCARE LTD
Biomarkers of chronic pelvic pain syndrome
Owner:NORTHWESTERN UNIV
Il-6 detection based early diagnosis and prediction of systemic inflammatory response syndrome and sepsis in asymptomatic patients
Owner:ROCHE DIAGNOSTICS OPERATIONS INC +1
Pro-adm as a therapy monitoring marker for critcally ill patients
ActiveCN111065927AReduce intensityReduce frequencyCompounds screening/testingDisease diagnosisTherapy monitoringCritically ill
The invention relates to a method for therapy monitoring, comprising the prognosis, risk assessment and / or risk stratification of a subsequent adverse event in the health of a patient, comprising providing a sample of said patient, wherein the patient has been diagnosed as being critically ill and medical treatment has been initiated, wherein the sample is isolated from the patient after diagnosisand treatment initiation; determining a level of proadrenomedullin (proADM) or fragment(s) thereof in said sample, wherein said level of proADM or fragment(s) thereof correlates with the likelihood of a subsequent adverse event in the health of said patient. In a preferred embodiment the invention relates to a method comprising additionally determining a level of procalcitonin (PCT) or fragment(s) thereof in a sample isolated from the patient. Preferably, a method of the present invention comprises determining a level of procalcitonin (PCT) or fragment(s) thereof in a first sample isolated from the patient, wherein said first sample is isolated at or before the time point of diagnosis and treatment initiation (time point 0); determining a level of PCT or fragment(s) thereof in a second sample isolated from said patient after diagnosis and treatment initiation; and determining whether a difference in the level of PCT or fragment(s) thereof in the second sample is evident in comparisonto the level of PCT or fragment(s) thereof in the first sample.
Owner:BRAHMS GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com