CRP monoclonal antibody nanometer latex microsphere composition and preparation process thereof
A monoclonal antibody and latex microsphere technology, applied in the field of medical immunity, can solve the problems of narrow linear range, inability to cover trace CRP, inability to detect CRP, etc., and achieve the effect of small difference, wide application and stable quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of CRP monoclonal antibody
[0036] 1 Separation and purification of human serum CRP
[0037] 1.1 Ammonium sulfate precipitation of total protein
[0038] Precipitate the total protein in human serum samples (CRP>100mg / L) with ammonium sulfate with a saturation of 80%, and precipitate overnight at 4°C; the next day, centrifuge at 8000rpm, 4°C for 30min, and wash with basic buffer (20mM Tris-HCl ,pH7.8) to dissolve the precipitate, fully dialyzed to remove ammonium sulfate.
[0039] 1.2 Primary purification of CRP
[0040] Take an appropriate amount of activated DEAE filler (Diethylaminoethyl cellulose) to pack into the column, and equilibrate with the above-mentioned basic buffer. Load the above-mentioned pretreated sample, and carry out gradient elution by changing the ionic strength (the NaCl concentration in the eluent is 80mM, 120mM, and 250mM respectively), collect the eluate respectively, and measure the protein concentration with a UV spectrophotom...
Embodiment 2
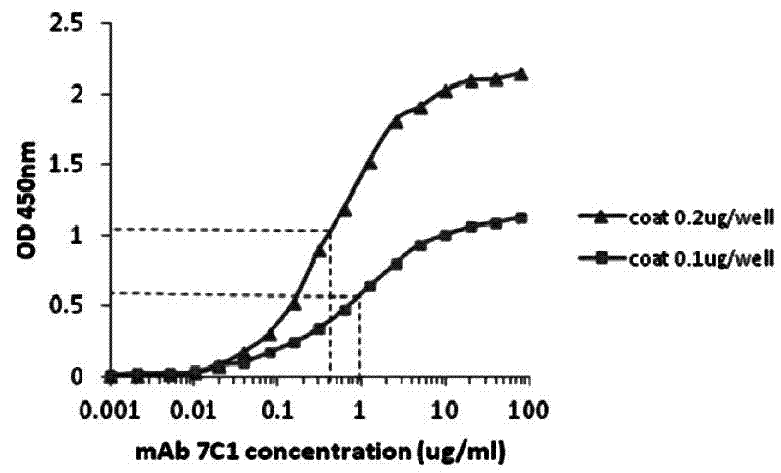
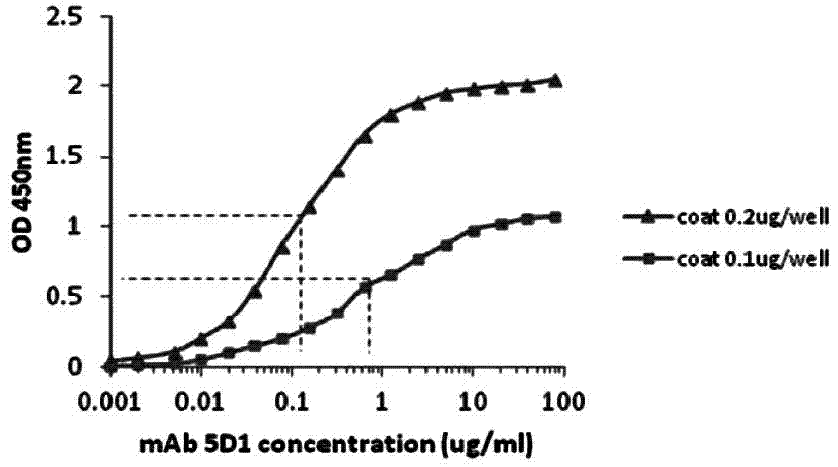
[0071] Determination of Affinity Constant of CRP Monoclonal Antibody by Direct ELISA
[0072] Experimental materials: mouse anti-human CRP monoclonal antibody, from the culture supernatant of CRP monoclonal antibody cell line, filtered through a 0.22 μm filter membrane, affinity purified by Sepharose Protein G, and filtered by Superdex 200 gel, and the isolated group of 200kD-100kD was collected After being concentrated to a certain concentration by ultrafiltration, it was filtered through a 0.22 μm filter membrane and stored in PBS buffer plus 20% (v / v) glycerol at -20°C. The titer determined by indirect ELISA was 2.5×10 6 .
[0073] Experimental procedure: use non-competitive enzyme immunoassay to detect monoclonal antibody affinity constant. The above-mentioned purified human CRP protein was plated and fixed at 0.1 μg and 0.2 μg per reaction well, respectively, using monoclonal antibodies 7C1 and 5D1 as primary antibodies, and horseradish peroxidase (HRP)-coupled rabbit a...
Embodiment 3
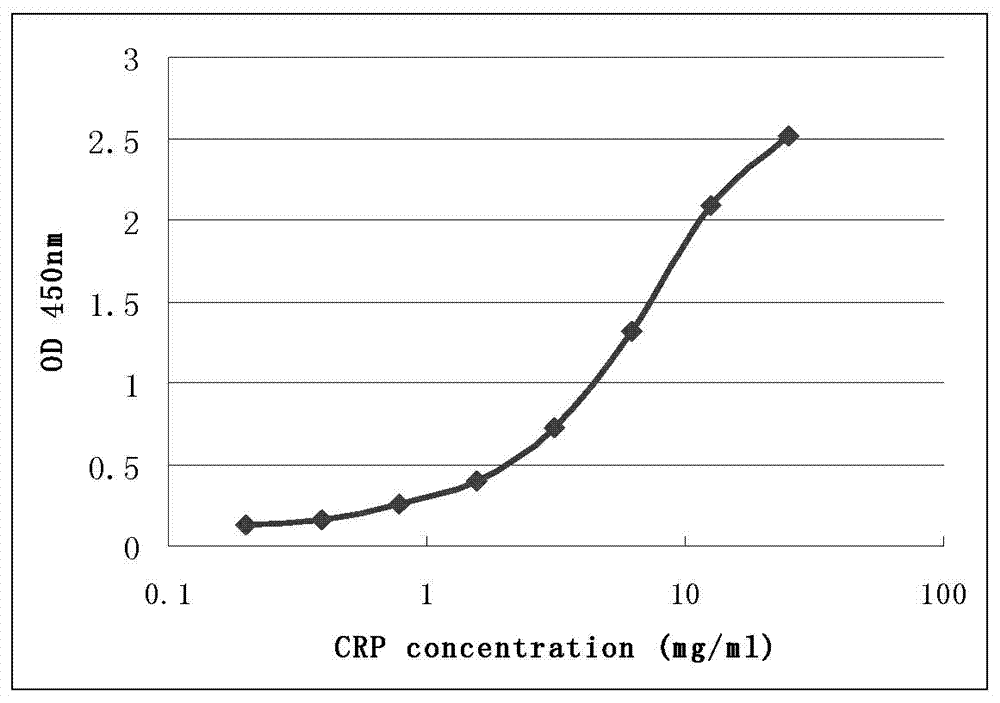
[0075] Identification of CRP monoclonal antibodies 7C1 and 5D1 with different epitopes by sandwich ELISA
[0076] Experimental material: see embodiment 2.
[0077] Experimental procedure: select two complementary monoclonal antibodies 7C1 and 5D1, and detect whether their antigenic epitopes are different. Each reaction well was coated with 0.2 μg monoclonal antibody 7C1 as a capture antibody on the solid phase carrier, and each reaction well was used as a detection antibody with 0.02 μg horseradish peroxidase (HRP)-coupled monoclonal antibody 5D1. Human C-reactive protein was purified and quantified by BCA method, then diluted to different concentrations with PBS buffer, and then detected by sandwich ELISA method. by OD 450nm Reading detection. The test results are shown in Table 1. Take the data in Table 1 as figure 2 . figure 2 Ordinate is OD 450nm The measured value of , the abscissa is the logarithmic value of different CRP antigen concentrations (mg / L).
[0078]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com