Pro-adm as a therapy monitoring marker for critcally ill patients
A patient-severity technology, applied in the field of therapy monitoring, that solves problems such as accurate measurement of disease severity that has not yet been revealed, improving medical care and avoiding costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
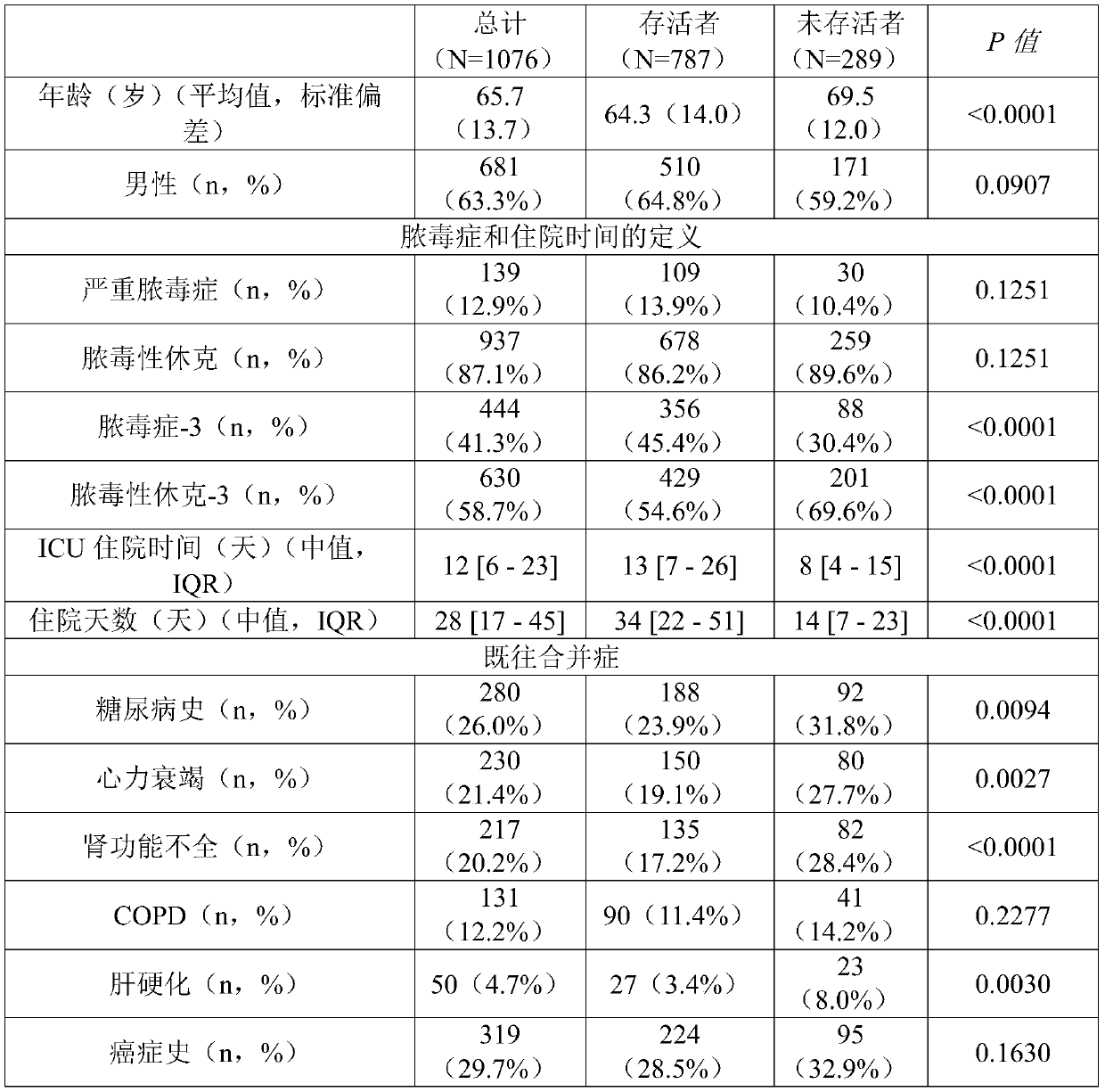
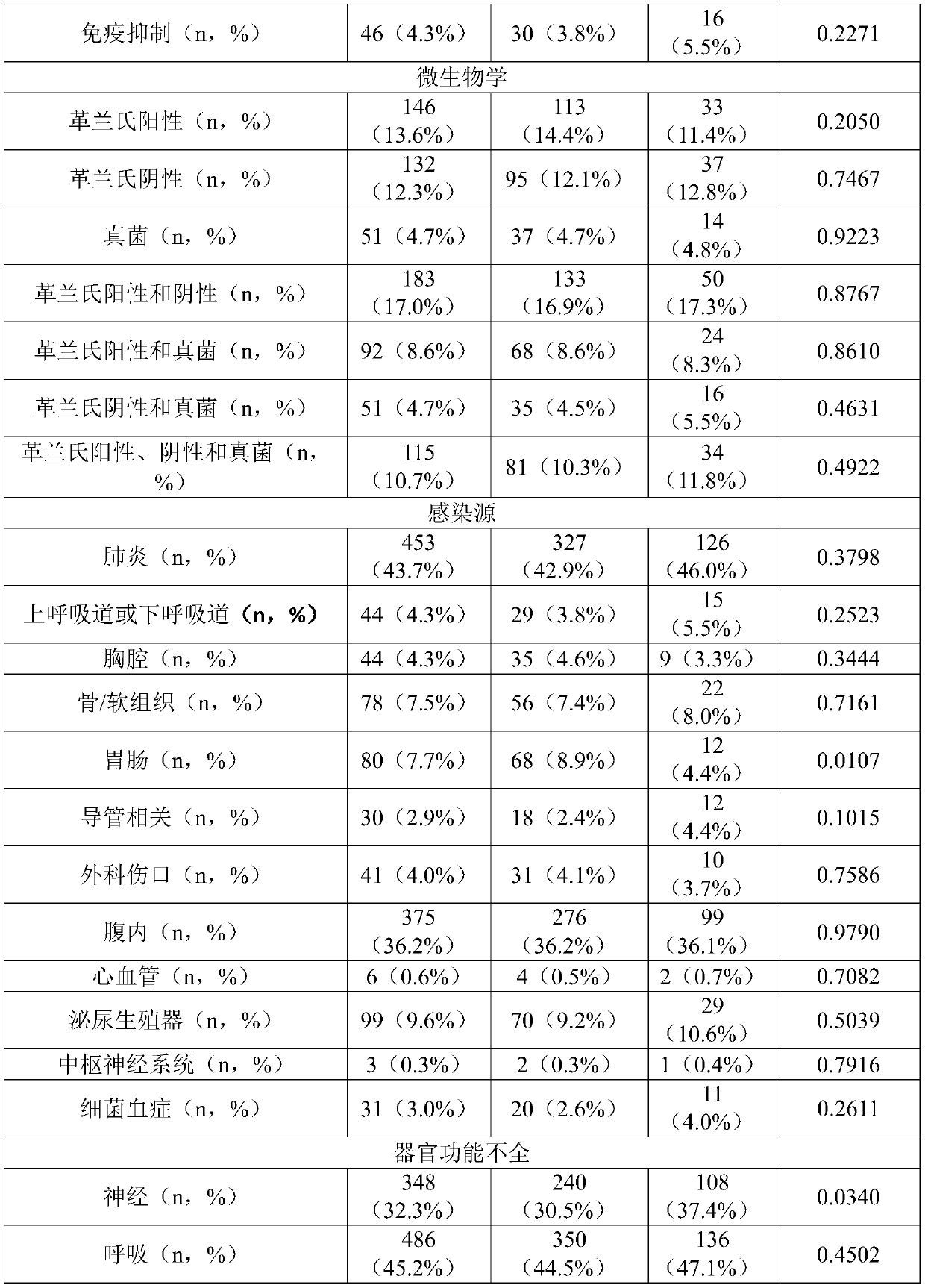
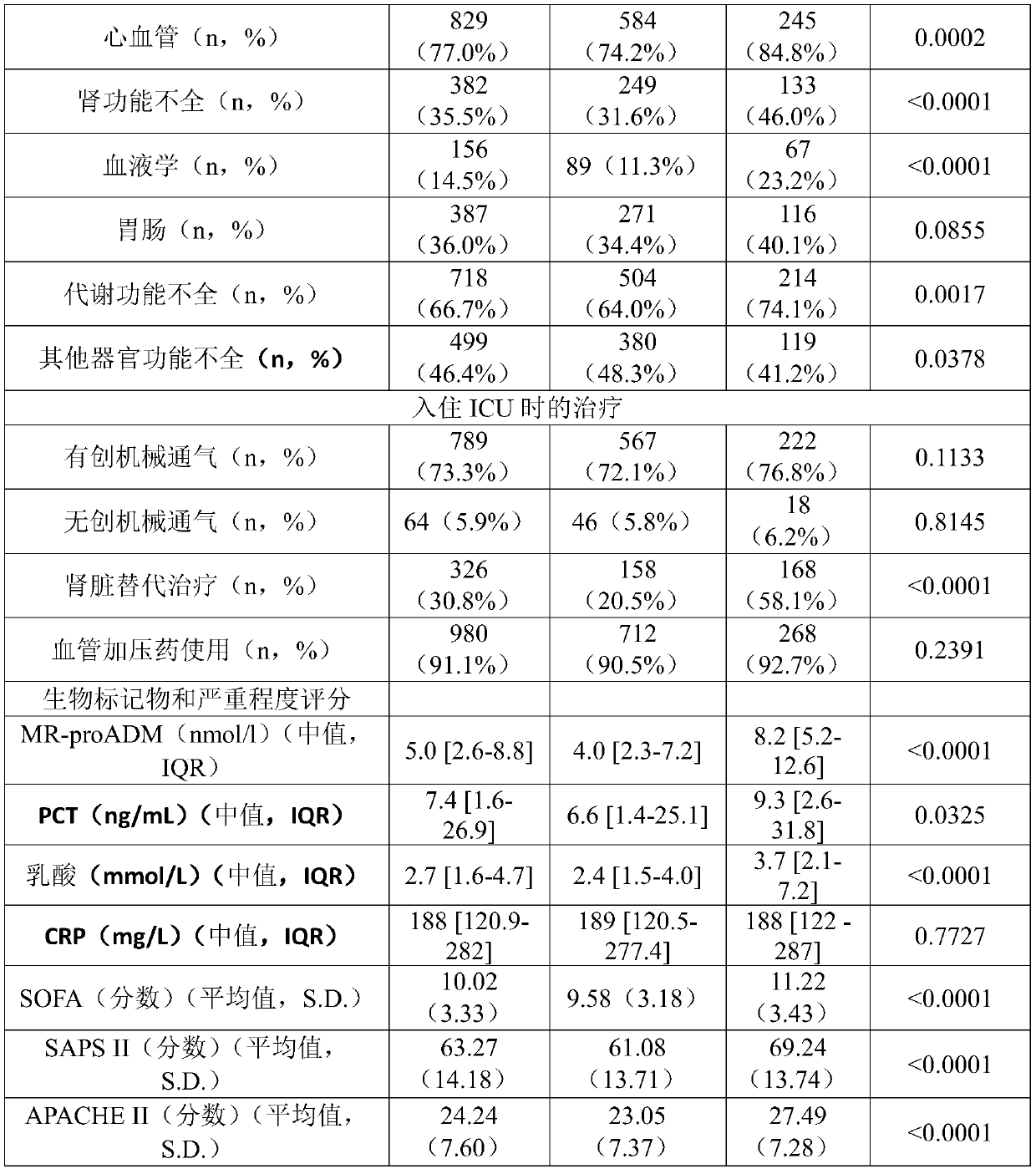
[0433] Example 1: Patient Characteristics
[0434] Table 1 summarizes patient characteristics at study enrollment.
[0435] A total of 1089 patients with severe sepsis (13.0%) or septic shock (87.0%) were analyzed, of which 445 (41.3%) and 633 (58.7%) patients also met sepsis-3 and septic shock-3, respectively standard. The mean age of the registered patients was 65.7 (13.7) years old, and the mean SOFA score was 10.0 (3.3) points. The 28-day all-cause mortality rate (N=1076) was 26.9% (sepsis-3: 20.0%; septic shock-3: 32.1%), and the in-hospital mortality rate was 33.4% (sepsis-3: 24.4%; Toxic shock-3: 40.4%). 836 patients (77.7%) were found to have infections originating from a single lesion, among them, pulmonary (N=324; 30.1%), intra-abdominal (N=252; 23.4%), genitourinary (N=57; 5.3%) and bone / soft tissue (N=50; 4.6%) origin were most prevalent. The corresponding mortality rates were 26.5%, 24.6%, 22.8% and 28.0%, respectively. Multiple sources of infection were fou...
example 2
[0436] Example 2: Relationship of Baseline Biomarkers and Clinical Scores to Mortality
[0437] Univariate and multivariate Cox regression analyzes found that MR-proADM was most strongly associated with 28-day mortality in the overall patient population and in the sepsis-3 and septic shock-3 subgroups (Table 2). The corresponding AUROC analysis found significant differences in all biomarkers and clinical scores compared with MR-proADM, except APACHE II (subgroup of patients with sepsis-3).
[0438] Similar results were found for 7-day, 90-day, ICU, and in-hospital mortality prediction (Table 3), with the addition of MR-proADM to all potential biomarker and clinical score combinations (N=63) significantly improving the prognostic power (Table 4).
example 3
[0439] Example 3: Identification of high-risk patients
[0440] The total patient population was further stratified according to existing SOFA severity levels, as well as biomarker and clinical score performance in predicting 28-day mortality assessed in each subgroup. MR-proADM showed the highest accuracy for all parameters in the low (SOFA ≤ 7) and moderate (8 ≤ SOFA ≤ 13) severity SOFA subgroups (Table 5; Table 6).
[0441] Two corresponding MR-proADM cutoffs were then calculated to identify low (≤2.7 nmol / l) and high (>10.9 nmol / l) severity subgroups at baseline. Compared with SOFA, it can be found in low (MR-proADM / SOFA: N=265 / 232; 9.8% / 13.8% mortality) and high (MR-proADM / SOFA: N=161 / 155; 55.9% / 41.3%) A more precise reclassification was performed under the severity cutoff (Table 7).
[0442] The subgroup of 94 patients (9.3%) with high MR-proADM concentrations and corresponding low or intermediate SOFA values had mortality rates of 57.4% and 68.9% at 28 and 90 days, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com