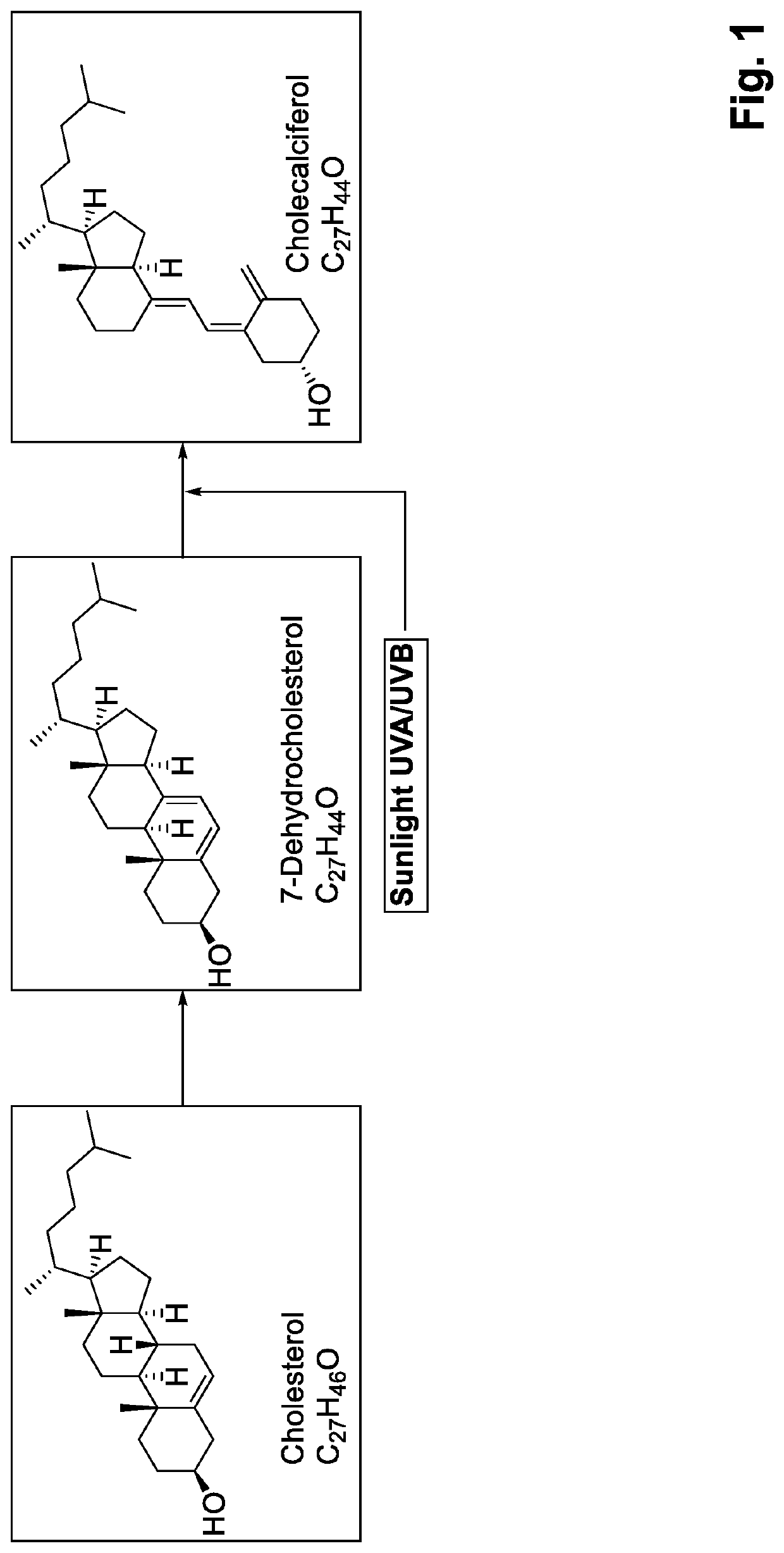
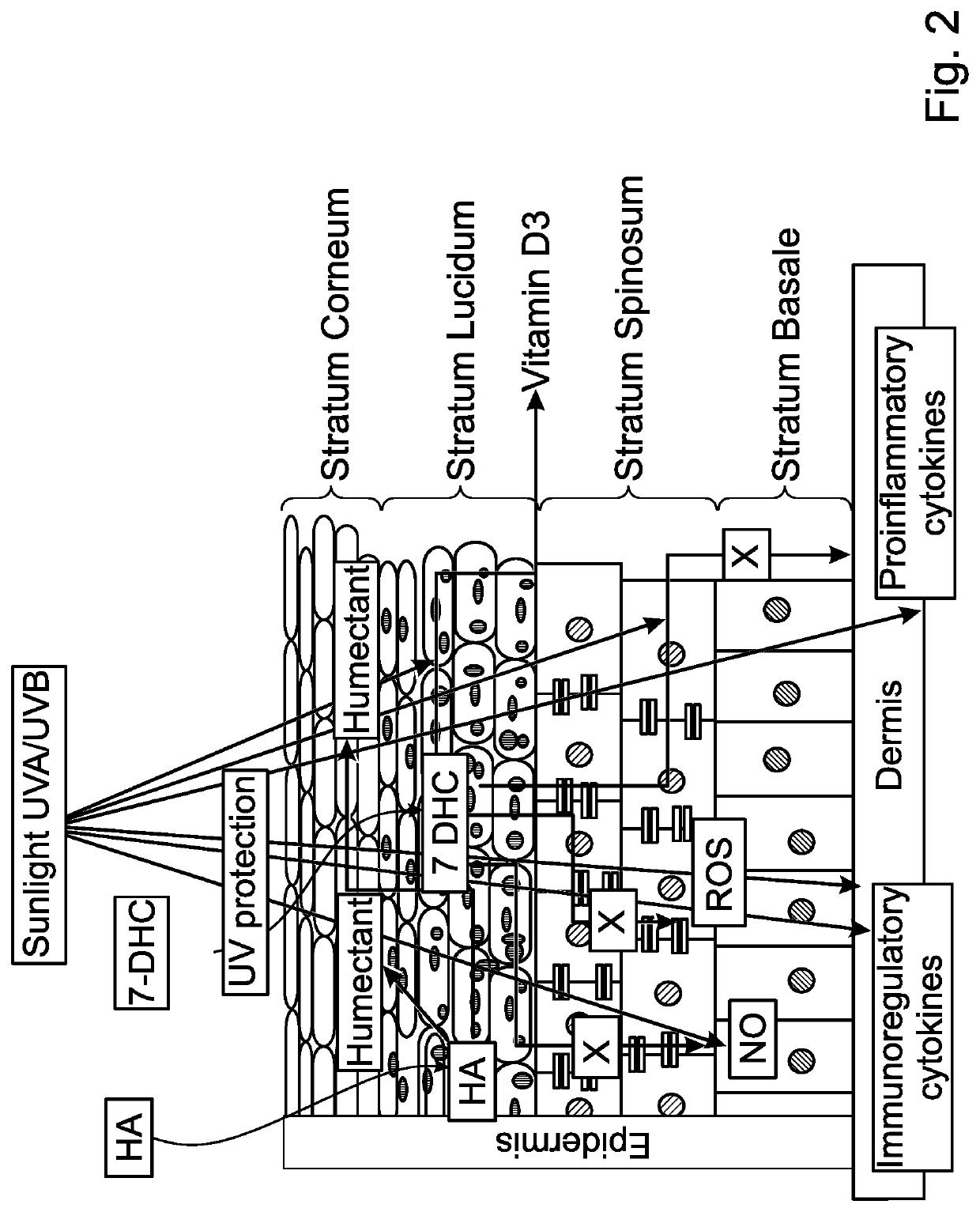
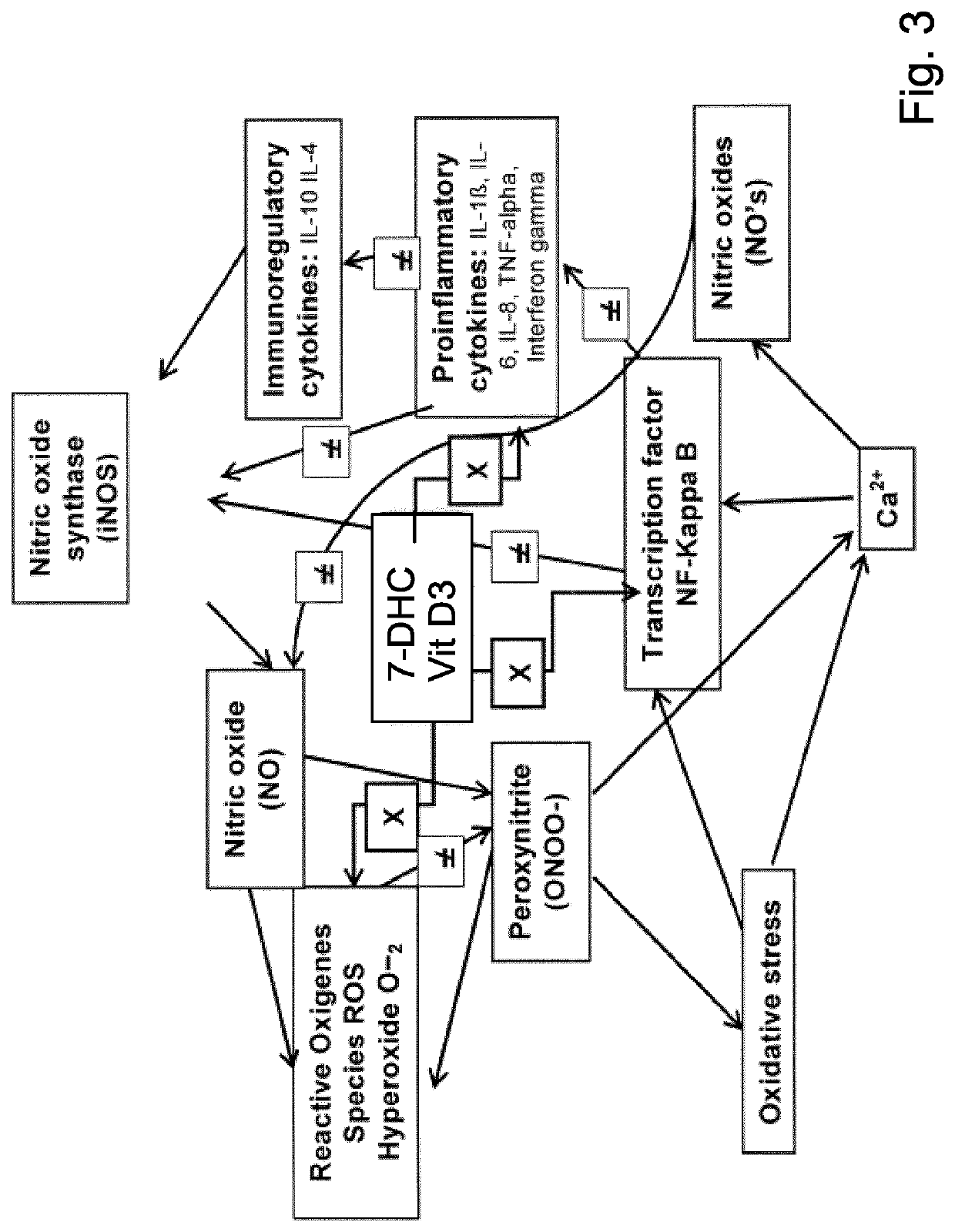
Pharmaceutical compositions
a technology of pharmaceutical compositions and compositions, applied in the field of pharmaceutical compositions, can solve the problems of skin cancer, damage to epidermal and dermal layers, and the size of the dermis layer, and achieve the effect of optimal skin penetration and stabilization of vitamin d3
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
without UV-Filter System Cream
[0080]
TABLE 4QuantityIngredients (INCI)wt %CAS-No.7-DHC0.15434-16-2HA39004-61-9Retinyl Palmitate0.579-81-2SkinConditioningRiboflavin0.183-88-5Niacinamide4.098-92-0Dexpanthenol2.581-13-0Folic acid0.0559-30-3L-ascorbic acid3.050-81-7Tocopheryl Acetate2.07695-91-2Aqua destillata46.1967732-18-5SolventPEG-5 Glyceryl Stearate1.051158-08-8Surfactant138860-92-1Stearic Acid1.057-11-44Emulsifying EmolientIsopropyl Myristate1.0110-27-0Palmitic Acid1.057-10-3Lupinus albus Ölextrakt2.0545-47-1Palmitoyl-Pentapeptid1.0214047-00-4Prunus amygalus dulcis oil5.08007-69-0 / 90320-37-9Squalene1.0111-02-4Polidocanol3.03055-99-0Hydroxypalmitoylsphinganin1.0190249-36-6Pyridoxine HCL5.08007-69-0 / Conditioning90320-37-9ConservantSodium Ascorbyl Phosphate2.066170-10-3Sodium Citrate1.06132-04-3BufferLecithin28002-43-5Carrierencapsulationformulary*Sphingomyeline285187-10-6Linolenic Acid1463-40-1Linoleic Acid160-33-3Phosphatidylcholin326853-31-6Caprylic Triglyceride173398-61-5Octyldode...
example 2
without UV-Filter System Gel
[0081]
TABLE 5QuantityIngredients (INCI)wt %FunctionCAS-No.7-DHC0.15Active ingredient434-16-2ROS binderHA3Active ingredient9004-61-9HumectantRetinyl Palmitate0.5Skin Conditioning79-81-2Riboflavin0.1Skin Conditioning83-88-5Niacinamide4.0Skin Conditioning98-92-0Dexpanthenol2.5Skin Conditioning81-13-0Folic acid0.05Skin Conditioning59-30-3L-ascorbic acid3.0Skin Conditioning50-81-7Tocopheryl2.0Skin Conditioning7695-91-2AcetateAqua destillata65.77Solvent7732-18-5Stearic Acid1.0Surfactant57-11-44Isopropyl1.0Emulsifying Emolient110-27-0MyristatePalmitic Acid1.0Emulsifying Emolient57-10-3Cetearyl Alcohol1.0Emulsifying Emolient67762-27-0 / 8005-44-5Polidocanol2.0Emulsifying Emolient3055-99-0Sodium Citrate1.0Buffering6132-04-3Lecithin1Carrier encapsulation8002-43-5formulary*Sphingomyeline2Carrier encapsulation85187-10-6formulary*Linolenic Acid1Carrier encapsulation463-40-1formulary*Linoleic Acid1Carrier encapsulation60-33-3formulary*Phosphatidylcholin2.0Carrier encapsu...
example 3
without UV-Filter System Serum
[0082]
TABLE 6QuantityIngredients (INCI)wt %FunctionCAS-No.7-DHC0.15Active434-16-2ingredientROS binderHA3Active9004-61-9ingredientHumectantDexpanthenol2.5Skin81-13-0ConditioningFolic acid0.05Skin59-30-3ConditioningTocopheryl Acetate2.0Skin7695-91-2ConditioningAqua destillata70.3Solvent7732-18-5PEG-5 Glyceryl Stearate1.0Surfactant51158-08-8138860-92-1Stearic Acid1.0Surfactant57-11-44Isopropyl Myristate1.0Emulsifying110-27-0EmolientPalmitic Acid1.0Emulsifying57-10-3EmolientPalmitoyl-Pentapeptid1.0Emulsifying214047-00-4EmolientPolidocanol2.0Emulsifying3055-99-0EmolientHydroxypalmitoylsphinganin1.0Emulsifying190249-36-6EmolientSodium Citrate1.0Buffering6132-04-3Lecithin1Carrier8002-43-5encapsulationformulary*Sphingomyeline2Carrier85187-10-6encapsulationformulary*Linolenic Acid1Carrier463-40-1encapsulationformulary*Linoleic Acid1Carrier60-33-3encapsulationformulary*Phosphatidylcholin3Carrier26853-31-6encapsulationformulary*Caprylic Triglyceride1Carrier73398-6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com