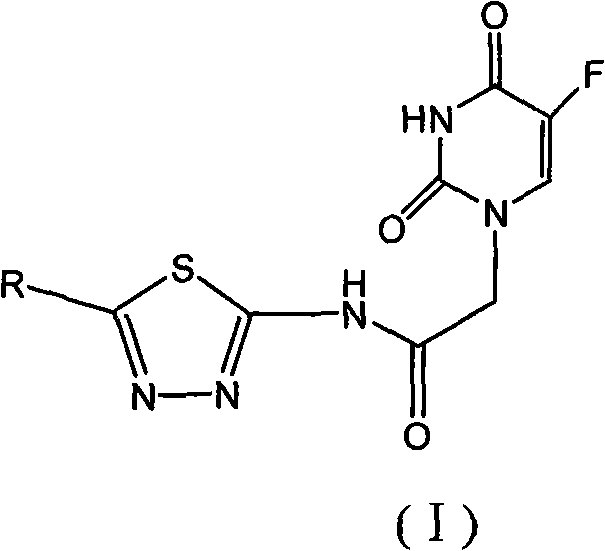
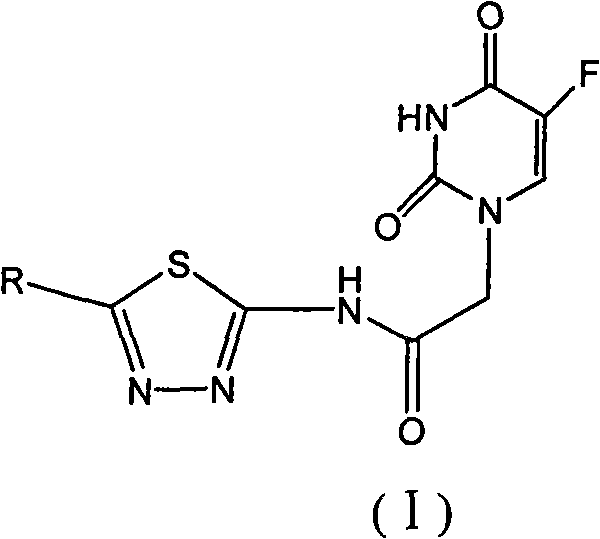
1,3,4-thiadiazole fluorouracil compound as well as preparation method and application thereof
A technology of compound and alkyl, which is applied in the field of pesticides, can solve the problems of increased risk and increased resistance of pests, and achieves the effects of low cost, overcoming the problem of drug resistance, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] This example illustrates the synthesis of 2-chloro-N-(5-phenyl-1,3,4-thiadiazol-2-yl)acetamide
[0061] Into a 250ml four-neck flask, add 0.01mol of 2-amino-5-phenyl-1,3,4-thiadiazole, 100ml of acetone, and 0.02mol of anhydrous potassium carbonate. 0.02mol of chloroacetyl chloride dissolved in 15ml of acetone was added dropwise under ice-cooling. After the dropwise addition was completed, the mixture was reacted at 20°C for 4 hours and filtered. The filter cake was acid-washed with 1mol / L HCl solution, 5% NaHCO 3 The solution was washed with alkali and washed with water to obtain a crude product. The product is obtained by recrystallization from ethanol, melting point: 220-223°C.
Embodiment 2
[0063] This example illustrates the synthesis of 2-(5-fluorouracil-1-yl)-N-(5-phenyl-1,3,4-thiadiazol-2-yl)acetamide.
[0064] Add 0.006mol of KOH, 15ml of water, and 0.005mol of 5-FU into a 100ml four-neck flask. The pH value of the solution was adjusted to 10 at 20°C, and 15 ml of a DMF solution containing 0.0036 mol of 2-chloro-N-(5-phenyl-1,3,4-thiadiazol-2-yl)acetamide was slowly added dropwise. After the dropwise addition, the temperature was raised to 120° C. for 3 hours to react. After cooling down to room temperature, a solid precipitated out and was filtered, and the crude product was recrystallized from DMF. A white flocculent solid was obtained. Yield 82%. Melting point: >300°C
[0065] 1 H NMR: 4.60(2H, s), 7.53(4H, s), 7.93~8.30(2H, m), 11.96(1H, s), 13.06(1H, s)
Embodiment 3
[0067] This example illustrates the synthesis of 2-chloro-N-[5-(3-methoxyphenyl)-1,3,4-thiadiazol-2-yl]acetamide
[0068] According to the method of Example 1, in a 250ml four-necked flask, add 2-amino-5-(3-methoxyphenyl)-1,3,4-thiadiazole 0.01mol, acetone 100ml, anhydrous potassium carbonate 0.02 mol. 0.02mol of chloroacetyl chloride dissolved in 15ml of acetone was added dropwise under ice-cooling. After the dropwise addition was completed, the mixture was reacted at 20°C for 3 hours and filtered. The filter cake was acid-washed with 1mol / L HCl solution, 5% NaHCO 3 The solution was washed with alkali and washed with water to obtain a crude product. The product is obtained by recrystallization with ethanol, melting point: 228-232°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com