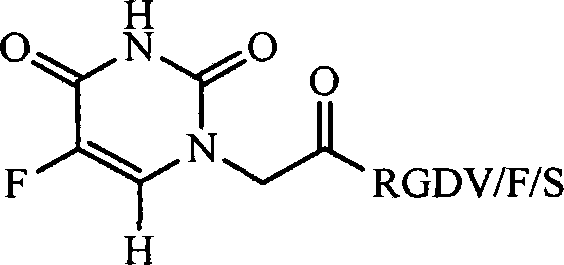
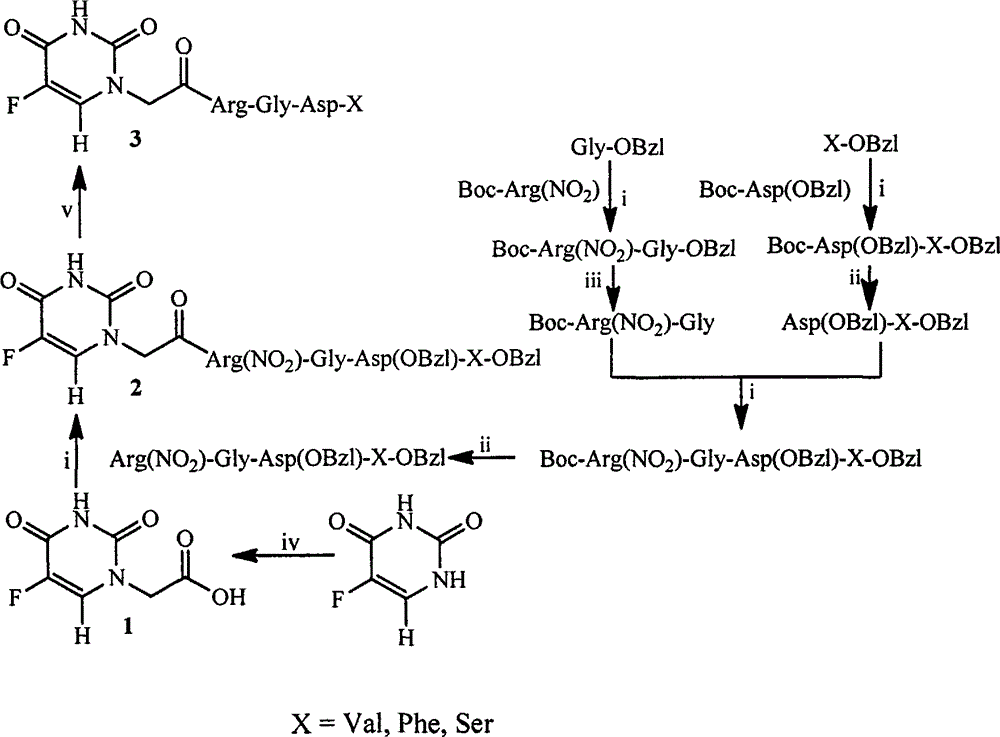
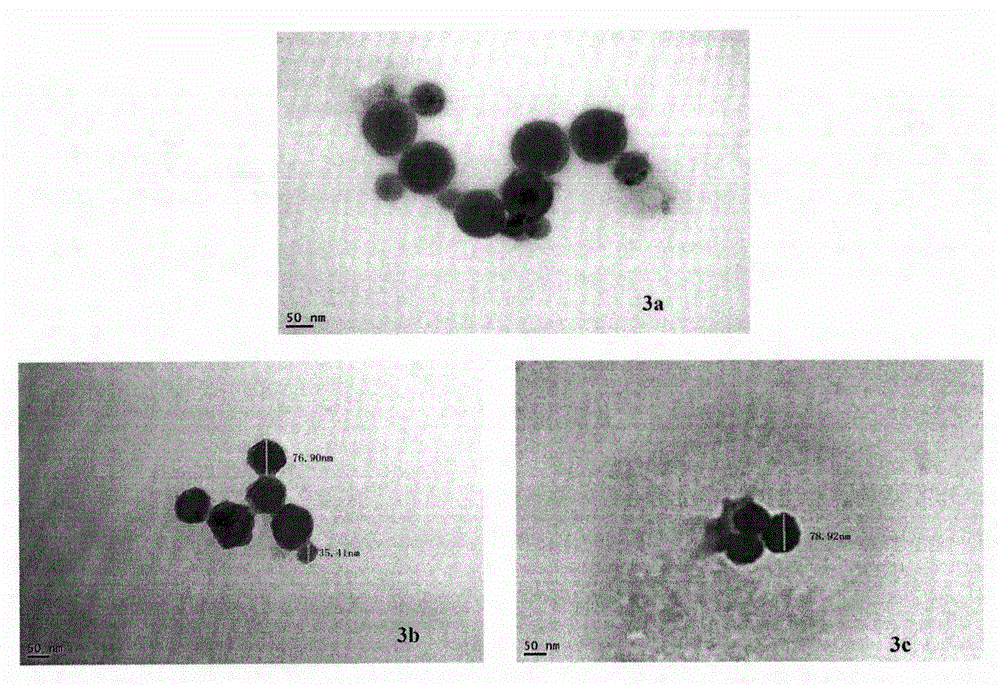
RGD modified 5-fluorouracil and preparation method, nanostructure, activity and application thereof
A technology of fluorouracil and f-obzl, applied in the field of biomedicine, can solve the problems of compounds with anti-tumor, anti-inflammation, anti-thrombotic and anti-tumor metastasis effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1 prepares 5-fluorouracil-1-base acetic acid
[0020] 1.30g (10mmol) of 5-fluorouracil was placed in a 100mL eggplant bottle, and 10mL of NaOH (30%) aqueous solution was added to dissolve it, and the reaction was heated up to 60°C. °C for 12 h, TLC (ethyl acetate: glacial acetic acid: water = 5:2:0.5) showed that the reaction was complete. Cool the reaction solution to room temperature, slowly add concentrated hydrochloric acid dropwise in an ice bath, adjust the pH to 2, stir for 0.5 h, a colorless solid precipitates, filter, wash the filter cake three times with ice water and diethyl ether, and dry in the shade. Yield 0.56 g (30%) of the title compound as a colorless powder. ESI-MS(m / e): 189[M+H] + . 1 HNMR (500MHz, DMSO-d 6 ): δ / ppm=13.21(s, 1H), 11.89(d, J=4.5Hz1H), 8.07(d, J=6.5Hz1H), 4.37(s, 2H).
Embodiment 2
[0021] Embodiment 2 prepares HCl Arg (NO 2 )-Gly-Asp(OBzl)-Val-OBzl
[0022] 1) Boc-Arg (NO 2 Preparation of )-Gly-OBzl
[0023] 19.94g (62.5mmol) Boc-Arg (NO 2 ) was dissolved in a 500mL reaction flask with 100mL of anhydrous THF, and 9.65g (68.75mmol) of N-hydroxybenzotriazole (HOBt) and 15.45g (75.0mmol) of dicyclohexylcarbodiimide (DCC ), stirred for 30min to obtain reactant A. Another 30.36g (85.53mmol) of Tos·Gly-OBzl was dissolved in 150mL of anhydrous THF in a 250mL reaction flask, and N-methylmorpholine (NMM) was slowly added dropwise in an ice bath to adjust the pH to 9 to obtain reactant B. Under ice bath, reactant B was added to reactant A, slowly added dropwise N-methylmorpholine (NMM) to adjust the pH to 9, stirred at room temperature for 12 h, thin layer chromatography TLC (dichloromethane: methanol: ice Acetic acid=20:1:0.1) showed that the reaction was complete. The reaction solution was filtered, the solvent was removed under reduced pressure, and the r...
Embodiment 3
[0034] Embodiment 3 prepares HCl Arg (NO 2 )-Gly-Asp(OBzl)-Phe-OBzl
[0035] 1) Boc-Arg (NO 2 Preparation of )-Gly-OBzl
[0036] Method is with embodiment 2 in 1).
[0037] 2) Boc-Arg (NO 2 )-Gly Preparation
[0038] Method is the same as 2) in embodiment 2.
[0039] 3) Preparation of Boc-Asp(OBzl)-Phe-OBzl
[0040] According to the operation of 1) in Example 2, by 22.10g (68.30mmol) Boc-Asp (OBzl), 10.10g (75.13mmol) N-hydroxybenzotriazole (HOBt), 16.90g (81.90mmol) bicyclic Hexylcarbodiimide (DCC) and 30.39 g (68.30 mmol) Tos·Phe-OBzl yielded 18.28 g (52.3%) Boc-Asp(OBzl)-Phe-OBzl as a colorless solid. ESI-MS(m / e): 561[M+H] + .
[0041] 4) Preparation of HCl Asp(OBzl)-Phe-OBzl
[0042] According to the operation of 4) in Example 2, 16.77g (91.2%) HCl·Asp(OBzl)-Phe-OBzl was prepared from 20.01g (35.74mmol) Boc-Asp(OBzl)-Phe-OBzl. ESI-MS(m / e): 459[M-H] - .
[0043] 5) Boc-Arg (NO 2 Preparation of )-Gly-Asp(OBzl)-Phe-OBzl
[0044] According to the operation of 1)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com