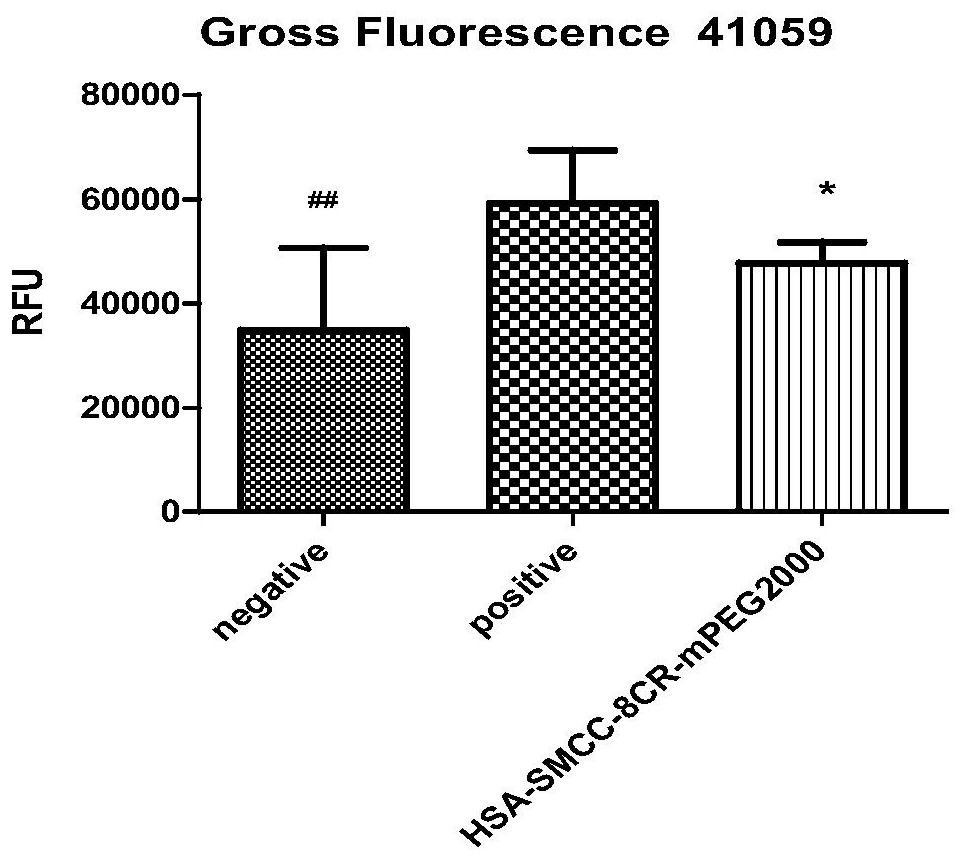
Preparation and application of surface double-modified targeting human serum albumin nano-drug carrier
A technology of human serum albumin and nano-drug carrier, which is applied in the direction of drug combination, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of lack of active targeting and improve the efficacy of drugs and safety, increase protein water solubility and stability, and improve the effect of plasma half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0047] Synthesis of Intermediate 2
[0048] Take 2 g of polyethylene glycol monomethyl ether with an average molecular weight of 2000 and dissolve it in 14 mL of distilled water, then add 1 mg of 2,2,6,6-tetramethylpiperidine oxide, 0.2 g of potassium bromide, stir in an ice bath, and then add 4.8 8% sodium hypochlorite solution, use 0.5N sodium hydroxide solution to adjust the pH of the system until the pH is stable at about 10, turn to room temperature and stir for 7 hours, add 0.7mL ethanol to annihilate the reaction, adjust the pH to 3 with 4N hydrochloric acid, and use dichloro Extracted with methane, dried over anhydrous sodium sulfate, spin-dried under reduced pressure, recrystallized with ether, and filtered to obtain 1.8 g of white solid.
Embodiment example 2
[0050] Synthesis of compound 8CR-mPEG2000
[0051] Take 0.674g resin octapeptide, add it to 7mL N,N-dimethylformamide, then add 1.9g intermediate 2, 358mg benzotriazole-N,N,N',N'-tetramethylurea hexa Fluorophosphate, 127mg 1-hydroxybenzotriazole, 0.31mL N,N-diisopropylethylamine, reacted at 30°C for 2.5h, detected by ninhydrin reaction method, the resin was colorless, and the reaction was complete. Wash the resin with 10 mL each of N,N-dimethylformamide, isopropanol, and N,N-dimethylformamide for 5 minutes in sequence and drain to obtain Resin-8CR-mPEG2000.
[0052] Preparation of lysate:
[0053] Phenol: ethanedithiol: sulfide anisole: water: trifluoroacetic acid = 5: 5: 2.5: 5: 82.5 (V / V)
[0054] Weigh 0.68g Resin-8CR-mPEG2000, add to 6ml lysate in ice bath, and react at 30°C for 2 hours. The reaction solution was filtered into cold diethyl ether and refrigerated overnight. After centrifugation, the resulting solid was washed three times with ether and dried with argon ...
Embodiment example 3
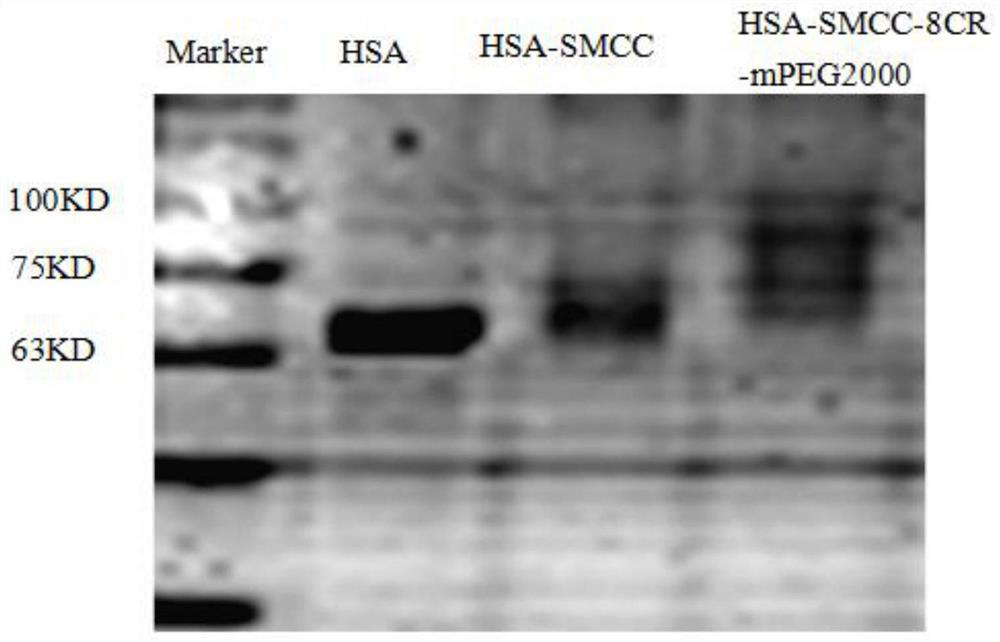
[0056] Synthesis of compound HSA-SMCC
[0057] Take 3.125mg of 4-(N-maleimidomethyl)cyclohexane-1-carboxylic acid sulfosuccinimide sodium salt, add 0.25mL of distilled water, stir at a temperature of about 40°C It was dissolved, and then 1.125 mL of PBS buffer solution was added, which was added to 8.94 mL of PBS buffer solution containing 20 mg of human serum albumin, protected by argon, and stirred at room temperature for 5 h. Dialyze in distilled water for 24 hours with a dialysis bag with a molecular weight cut-off of 8000-14000, and freeze-dry to obtain 20 mg of a white solid.
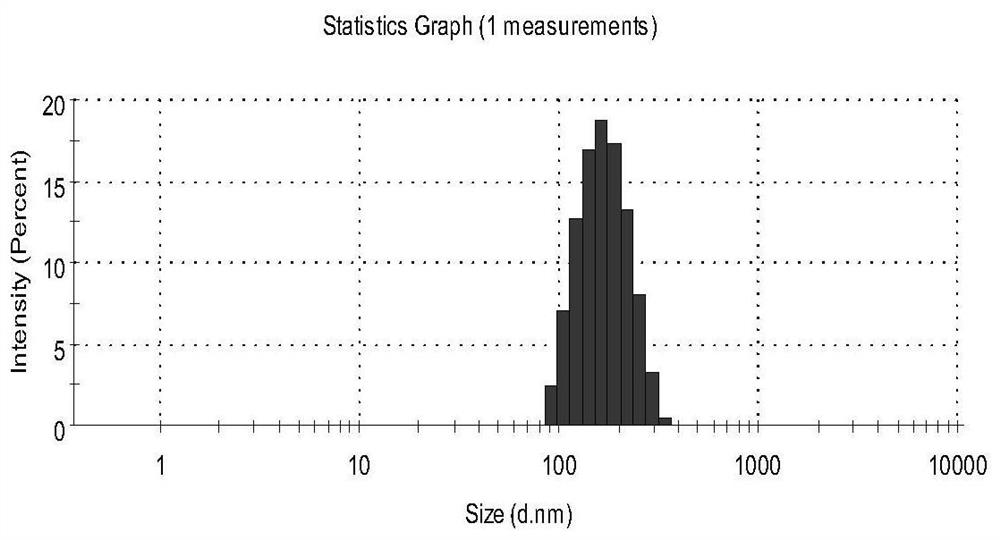
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com