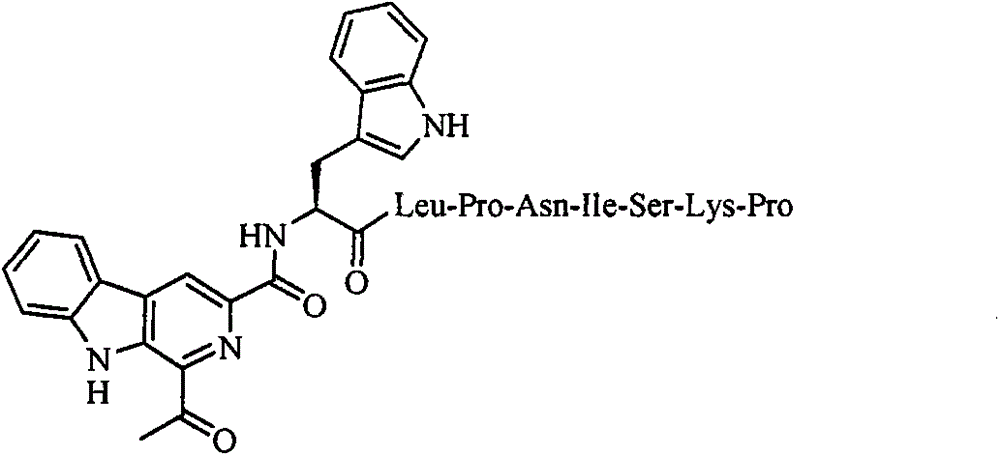
LPNISKP modified 1-acetyl-beta-carboline acyl-tryptophan as well as preparation method, nanostructure, activities and applications thereof
A technology of acetyl and carbolinyl, applied in the application of anti-tumor cell migration, adhesion and invasion drugs, in the field of anti-tumor cell adhesion, invasion and migration, can solve the problem of anti-tumor and anti-tumor adhesion, infiltration and Migration compounds etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
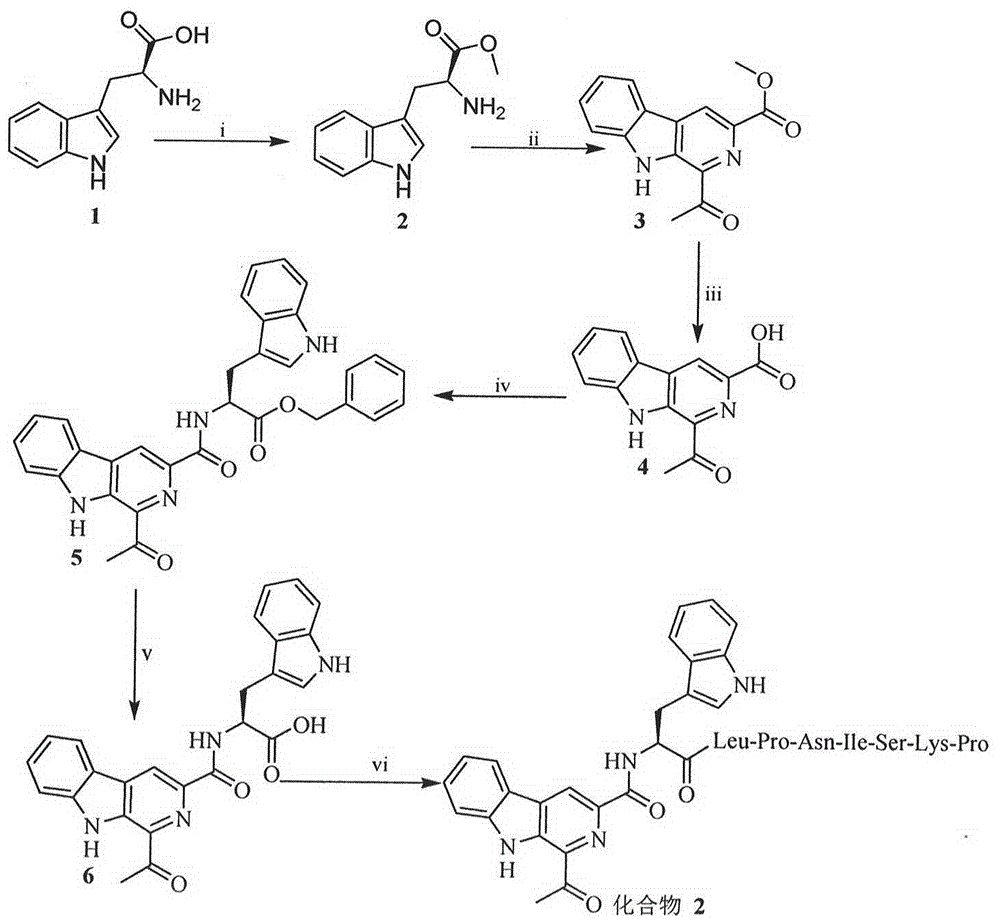
[0019] Example 1 Preparation of 1-acetyl-β-carboline-3-carboxylic acid methyl ester
[0020] Weigh 5g of L-Trp-OMe into a 100ml eggplant bottle, add 10ml of 37°C warm water and stir to dissolve, then add 3.3g of 1,3-dihydroxyacetone. React at room temperature for 36 h, centrifuge, discard the aqueous layer, and dry the precipitate to obtain 1 g (yield 20%) of the title compound as a brown solid, ready to use. ESI-MS(m / e): 257[M+H] + .
Embodiment 2
[0021] Example 2 Preparation of 1-acetyl-β-carboline-3-carboxylic acid
[0022] Weigh 5g of methyl 1-acetyl-β-carboline-3-carboxylate into a 250ml eggplant bottle, add dioxane to dissolve, slowly add 2N NaOH aqueous solution in an ice bath to adjust the pH to 12, and react for 13h. The reaction solution was saturated KHSO 4 The aqueous solution was adjusted to pH 8 and concentrated under reduced pressure. The residue was washed with saturated KHSO 4 The pH was adjusted to 2, the organic layer was extracted 3 times with ethyl acetate and concentrated under reduced pressure to afford 4 g (80%) of the title compound as a tan solid. ESI-MS (m / e): 241 [M-H] - .
Embodiment 3
[0023] Example 3 Preparation of 1-acetyl-β-carbolinyl-tryptophan benzyl ester
[0024] Weigh 2g of 1-acetyl-β-carboline-3-carboxylic acid, dissolve it in 10ml of anhydrous THF, add 2g of DCC and 1.2g of HOBt under ice cooling, and stir for 30min to obtain solution A. 3.2 g of Trp-OBzl was dissolved in 15 ml of anhydrous THF, and N-methylmorpholine was slowly added to adjust the pH of the solution to 9 to obtain solution B. Slowly add solution B into solution A under ice-cooling, adjust the pH to 9 with N-methylmorpholine, react for 12 hours, TLC shows that the raw material disappears (petroleum ether: acetone = 4:1). The reaction mixture was filtered, the filtrate was concentrated under reduced pressure, the residue was extracted 3 times with ethyl acetate, and concentrated under reduced pressure, the obtained 4.38g syrup product was purified by dry column chromatography of petroleum ether / acetone system (petroleum ether / acetone=4 / 1 ) afforded 2 g (46%) of the title compound ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com