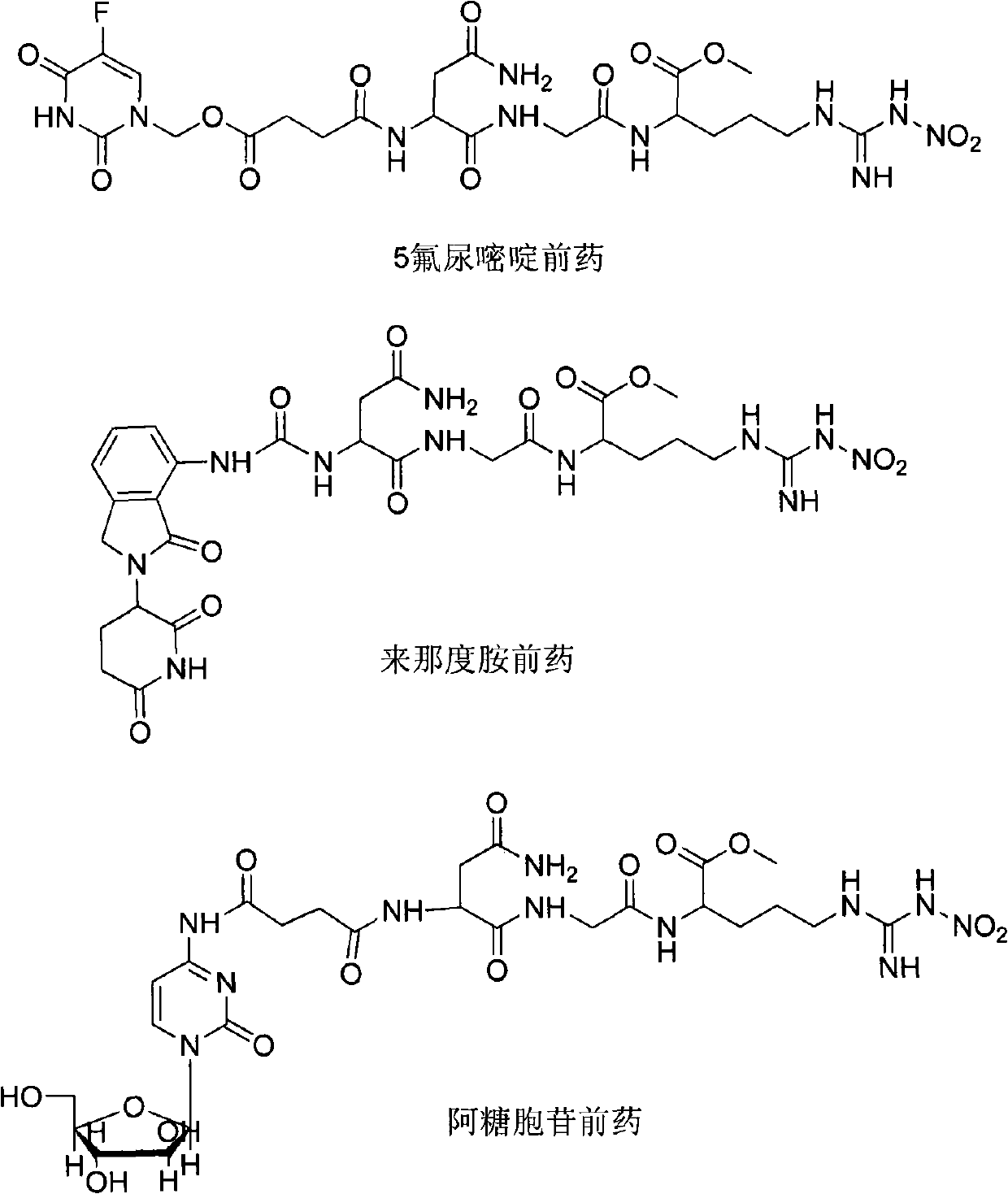
Novel anti-cancer medicaments using NGR(NO2) as targeting carrier, preparation thereof and use thereof
An anti-tumor drug and pharmaceutical technology, applied in the direction of anti-tumor drugs, drug combinations, medical preparations of non-active ingredients, etc., can solve problems such as short metabolic half-life, achieve the effect of reducing toxic side effects and improving targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
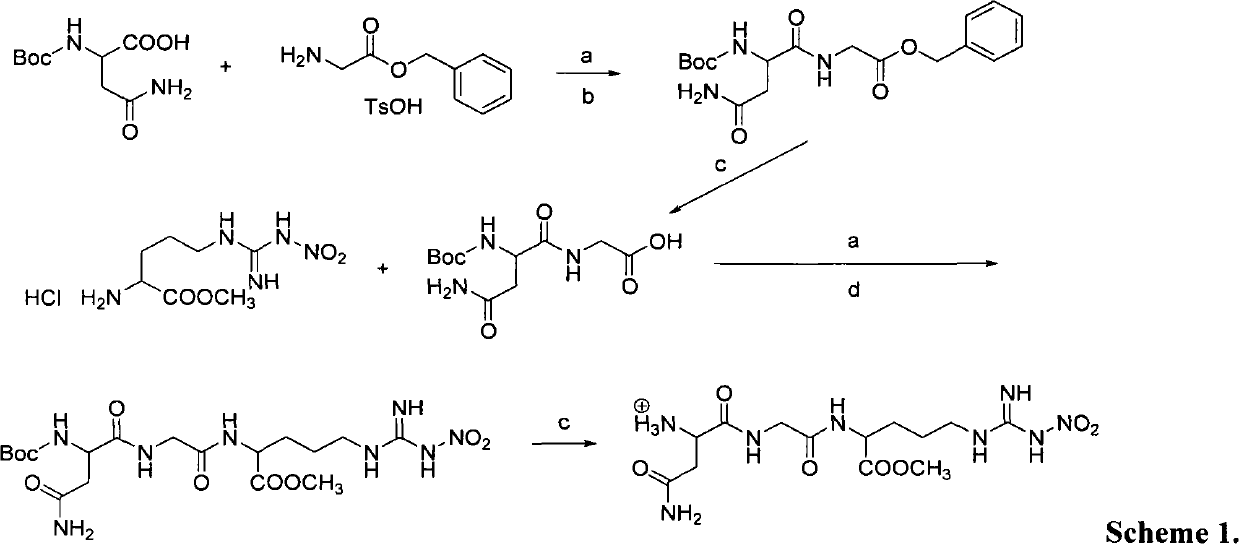
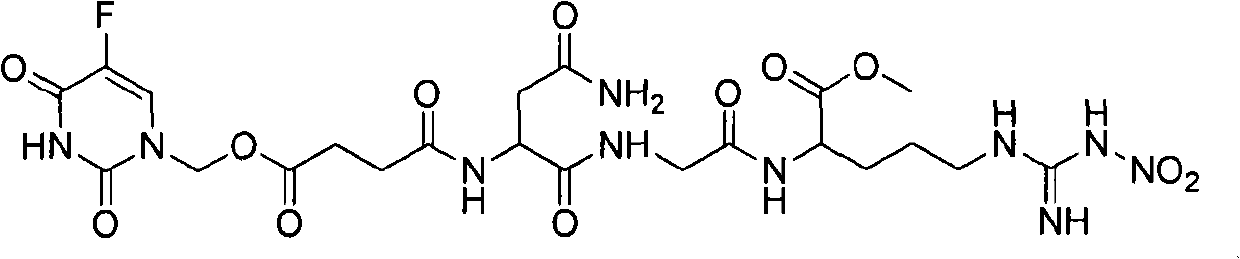
[0032] 5-FU-NGR (NO 2 The chemical synthesis of:
[0033] 1. Synthetic route:
[0034] 1.1NH 2 -Asn-Gly-Arg (NO 2 ) COOCH 3 Synthesis:
[0035]
[0036] Reagents and conditions: (a) HOBt, DCC, 0°C for 2h and then 8h at r.t.; (b) NH 2 -Gly-OBn TsOH, TEA.; (c) HCl-dry EtOAc.; (d) NH 2 -Arg(NO 2 )-COOCH 3 HCl, TEA.
[0037] 1.2.5-Fu-linker-Asn-Gly-Arg (NO 2 )-COOCH 3 Synthesis:
[0038]
[0039] Scheme 2. (a) 60℃.; (b) DCC, DMAP, dry THF.; (c) 10% Pd / C, AcOH, 1,4-cyclohexene.; (d) pentafluorophenol, EDAC, dry DMF.; (e) TEA, dry THF.
[0040] 2 synthesis method
[0041]Synthesis of Boc-protected asparagine-glycine benzyl ester Boc-Asn-Gly-OBn: 8.25 g (50 mmol) of Boc-protected asparagine was dissolved in 250 ml of anhydrous THF, and then 6.75 g (50 mmol) was added ) of HOBt and 10.3 g (50 mmol) of DCC, the mixed solution was stirred under ice bath for 1 hour, then the ice bath was removed, and the reaction was continued for 6 hours. An anhydrous tetrahydrofur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com